Lifestyle strategies to improve population cardiometabolic health remain a high priority as cardiovascular disease (CVD) continues to be a leading global health challenge(Reference Roth, Mensah and Johnson1). Clinical guidelines for prevention of heart disease are currently the same for men and women(2), despite evidence suggesting that there are sex differences in risk profiles and divergent physiological changes across the lifespan that may influence the impact of generic recommendations(Reference Pinho-Gomes, Peters and Thomson3). This is particularly relevant to the roles that dietary long-chain n-3 polyunsaturated fatty acids (LCn-3 PUFA) play in influencing risk of CVD, due to interactions with reproductive hormones and female-specific CVD risk factors, to be discussed later. This review critically examines the evolving body of knowledge concerning the impact of LCn-3 PUFA intake on CVD prevention in women, traversing the diverse stages of their lives from adolescence to postmenopausal years. The aim of this review is to summarise our understanding of the cardiovascular health impact of LCn-3 PUFA intakes across the female life course and to recommend priority areas for future research.
The impact of CVD on women
Research on the prevention and management of CVD in women has been comparatively neglected by the research community. However, awareness is increasing that CVD research does not predominantly belong in the domain of men’s health(Reference Mosca, Hammond and Mochari-Greenberger4). In the global female population, CVD is the primary cause of death, accounting for 35 % of mortality(5). Although CVD deaths in both sexes have been declining in countries with a high socio-demographic index (SDI), CVD remains responsible for a high proportion of deaths (34 % in women, 30 % in men across all high SDI countries). Furthermore, the proportion of deaths from CVD is increasing in low and middle SDI countries(5).
Worryingly, the prevalence of cardiovascular morbidity in younger women is increasing year on year. For example, data from a UK representative primary care database showed that whilst incidences and prevalences of diagnosed coronary heart disease (CHD) and angina were decreasing in women aged 16–50 years, there was an upward trend in myocardial infarction (MI) and revascularisation procedures from 1998 to 2017(Reference Okoth, Crowe and Marshall6). A Lancet report on women and CVD (2021) was commissioned on the basis that ‘CVD in women remains understudied, under-recognised, underdiagnosed and undertreated’(Reference Vogel, Acevedo and Appelman7), with the article culminating in key recommendations to reduce the global burden by 2030.
Differential impacts of cardiovascular risk factors in women
Many of the traditional modifiable risk factors for heart disease have been reported to confer a higher relative risk in women compared to men. Although these data are limited by the lack of representation of populations from non-European regions, they indicate that sex-specific cut-offs for risk factors in addition to waist and HDL cholesterol in clinical guidelines may facilitate better identification of women at increased risk of CVD. Obesity is one of these risk factors that confers a greater risk of CVD in women than men. For example, the age-adjusted relative risk of CVD associated with obesity was 1·64 (1·37–1·98) in women compared with 1·46 (1·20–1·77) in men in the Framingham heart study prospective cohort(Reference Wilson, D’Agostino and Sullivan8) and the hazard ratio for cardiovascular mortality in a German prospective cohort was 1·70 (1·27–2·28) in the highest v. lowest quartile for waist circumference for women compared with 1·28 (1·04–1·57) in men(Reference Rost, Freuer and Peters9). A higher waist-hip ratio increased risk of a CVD event across all BMI categories in women but not men in a Swedish cohort(Reference Li, Engström and Hedblad10), which is particularly pertinent to risk in women at midlife and beyond since the prevalence of central obesity is higher in postmenopausal women(Reference Banack, Bea and Chen11). In addition, the greater relative risk of CVD in women with obesity is compounded by the fact that a significant proportion of women experience gestational weight gain and post-partum weight retention(Reference Lipsky, Strawderman and Olson12).
Hypertension increases CVD risk more in women than men, accounting for 36 % of risk of MI among women v. 20 % of risk among men in the INTERHEART case-control study(Reference Yusuf, Hawken and Ounpuu13). In a study of combined prospective cohorts where blood pressure measurement was standardised, CVD risk increased at a lower blood pressure threshold in women compared to men(Reference Ji, Niiranen and Rader14), leading to suggestions that sex-specific ranges for normal and raised blood pressure are needed for assessing CVD risk. In addition, women experience an accelerated age-related increase in blood pressure in later life(Reference Pearson, Morrell and Brant15). Fasting blood triglycerides are more strongly independently associated with risk of CHD in women compared with men(Reference Castelli16). Non-fasting triglycerides are superior to fasting triglycerides in predicting risk of CHD and are also associated with a greater increase in risk of myocardial infarction in women compared to men, according to data from a Norwegian cohort(Reference Egeland, Igland and Sulo17).
Convincing evidence shows that the relative risk of CVD in people living with diabetes is greater in women than men(Reference Wang, O’Neil and Jiao18–Reference Peters, Huxley and Woodward21). These sex differences may be linked to the fact that women carry excess adiposity for a longer duration before the pathology of insulin resistance and impaired insulin secretion tips into overt type 2 diabetes, which renders them exposed to other cardiovascular risk factors for a longer period(Reference Logue, Walker and Colhoun22). In addition, a lack of understanding of sex-specific responses to therapies and disparities in clinical management are likely to play an important role(Reference Huebschmann, Huxley and Kohrt23). Other intertwining risk factors such as demands of domestic/family roles traditionally shouldered by women result in more limited opportunities for physical exercise. Lower physical activity levels may then interact with other risk factors such as hypertension and diabetes to amplify the heightened vascular risk associated with being female with these cardiovascular risk factors(Reference Guthold, Stevens and Riley24). Finally, depression is associated with a greater risk of CVD in women compared with men(Reference Lee, Yun and Ko25), and since women have a greater likelihood of developing depression, poor mental health is another important CVD risk factor that impacts women disproportionately.
Longer chain n-3 PUFA: sex-specific dietary recommendations and intakes
Dietary longer chain n-3 PUFA originate in marine microalgae that can carry out de novo synthesis of highly unSFA from SFA, including EPA(EPA; 20 : 5n-3), docosapentaenoic acid (DPA; 22 : 5n-3) and DHA (DHA; 22 : 6n-3). These LCn-3 PUFA become concentrated at each stage in the food chain, making fish a rich source of EPA, DPA and DHA. The other major n-3 fatty acid in the diet, the 18-carbon chain essential fatty acid, α-linolenic acid (ALA; 18 : 3n-3), is derived from plant foods (particularly vegetable oils, walnuts and seeds). ALA can be converted in small proportions to EPA, then DPA, and to a very limited extent to DHA in the body(Reference Harwood26).
An abundance of evidence points to the beneficial effects of LCn-3 PUFA on the pathological pathways that underlie CVD. The mechanisms mediating cardioprotective effects include those related to their preferential incorporation into cellular membranes, which may reduce risk of sudden cardiac death by lowering heart rate(Reference Mozaffarian, Geelen and Brouwer27), improving cardiac autonomic function(Reference Xin, Wei and Li28,Reference Christifano, Chollet-Hinton and Mathis29) and modulating ion channel function and cell signalling pathways(Reference Song, Dong and Wang30). Other pathways involved their direct effects on vasodilators in the vasculature(Reference Bercea, Cottrell and Tamagnini31), inhibition of inflammatory molecules and conversion to less proinflammatory eicosanoids and pro-resolving mediators that dampen and resolve inflammation(Reference Calder32), also by inhibiting platelet aggregation at higher doses(Reference Gao, Cao and Mao33), and by increasing lipoprotein lipase expression and activity(Reference Khan, Minihane and Talmud34), lipid oxidation(Reference Tian, Wang and Sun35) and decreasing de novo lipogenesis via modulation of liver transcription factors that regulate expression of lipogenic enzymes(Reference Kajikawa, Harada and Kawashima36,Reference Botolin, Wang and Christian37) , leading to a reduction in blood triglycerides(Reference Wang, Zhang and Zhou38).
Oily fish is the main source of dietary LC n-3 PUFA (on average 2·8 g per 140 g portion)(39), with small amounts derived from non-oily fish (0·4 g per portion)(39), eggs (0·1–0·2 g per egg)(40,Reference Kralik, Kralik and Košević41) and grass-fed beef/lamb (0·03–0·04 g per 100g)(40,Reference Scollan, Price and Morgan42) . Clearly, other nutritional benefits of fish exist apart from fatty acid composition: they are a good source of high-quality protein and micronutrients that are in limited supply elsewhere in the diet, including vitamin D, selenium and iodine. It is also important to consider that the total effects of fish consumption on health may be modified by other potentially harmful factors such as less favourable cooking methods(Reference Nahab, Pearson and Frankel43) and marine pollutants(39).
Recommendations in the UK, originating from the 2004 SACN report(39), are to consume at least 2 portions of fish/wk (at least 1 of which should be oily). Female-specific recommendations were included, for example, ‘Pregnant women, women intending to become pregnant, and under-16’s should avoid shark, marlin and swordfish, but can eat up to 4 medium-sized tins tuna or 2 tuna steaks per week’ when considering risks of methylmercury contamination, and ‘women past reproductive age, boys and men should consume 1–4 portions oily fish a week – women of reproductive age and girls 1–2 portions oily fish a week’ when considering risks of dioxins(39). However, representative data from the National Diet and Nutrition Survey Y1-11 indicates that the UK population fall far short of meeting recommendations. Over the whole survey period from 2008 to 2019 (n 7207 males and n 8448 females) only 11·7 % of females and 10·7 % of males of all ages met government recommendations to consume 1 portion of oily fish per week (estimated as >20 g/d from 4 d food diaries). There were no discernable changes in oily fish intakes over time in either sex (own analysis, National Diet & Nutrition Survey (NDNS) Y1-11)(44). These data suggest that most of the UK population are at risk of suboptimal tissue EPA and DHA status. Further analysis by age group reveals that 87·4 % and 87·9 % of females and males, respectively, under the age of 18 years, reported consuming no oily fish (Table 1)(44). Only 4·2 % of females and 4·7 % of males under-eighteens reported that they consumed the recommended amount. The proportion of women consuming oily fish was higher with age, with up to one-quarter of 66–80 year old females meeting guidelines, and a further 10·9 % consuming some oily fish (Table 1)(44). Concerningly, more than three-quarters of women may not be consuming any oily fish during child-bearing years (Table 1).
Table 1. Percentages of females and males meeting recommendations for consuming oily fish (1 portion per week, estimated at >20 g/d) by age category using 4-day food diary data from the National Diet and Nutrition Survey Rolling Programme Years 1–11 for 15 655 individuals

n-3 PUFA status through the female life course
Using red blood cell (RBC) membrane profiles as a proxy for tissue status, it can be determined that there are sex-specific variations in tissue omega-3 fatty acid status across the lifecourse(Reference Harris, Pottala and Varvel45). Data from 160 000 blood samples sent to a commercial clinical laboratory showed that the proportion of RBC lipids as ALA was higher in women than men across all ages whereas the proportion as EPA was lower in women until their 50’s(Reference Harris, Pottala and Varvel45). The proportion of RBC lipids as DHA increased with age in both sexes, but was higher in female adolescents and women in their twenties than men of equivalent age(Reference Harris, Pottala and Varvel45). These sex differences in tissue pools may reflect differences in conversion of EPA to DHA in pre-menopausal women. The conversion of ALA to EPA and then to DHA is known to be enhanced by oestrogen, as indicated by stable isotope-based research showing that the rate of conversion of labelled ALA to EPA was 8 % in men and 21 % in women (whose average age was 28 years), and then further conversion to DHA was <1 % in men and 9 % in women(Reference Burdge and Wootton46,Reference Burdge, Jones and Wootton47) . Women on oral oestrogenic contraceptives have higher circulating DHA concentrations(Reference Magnusardottir, Steingrimsdottir and Thorgeirsdottir48–Reference Giltay, Gooren and Toorians50), although this might not be true for all ethnic groups(Reference Abdelmagid, Clarke and Roke51). Postmenopausal women taking hormone replacement therapy (HRT) have higher erythrocyte DHA compared with those not on HRT(Reference Harris, Tintle and Manson52). Research in animal models has shown that oestrogen enhances PPARα activity leading to increased expression of the fatty acid desaturase 2 (FADS2) enzyme which desaturates ALA and DPA, and elongases, which increase the chain length along the conversion pathway(Reference Kitson, Marks and Shaw53).
After the menopause, oestrogen levels rapidly decline leading to documented increases in CVD risk factors including raised blood pressure(Reference de Kat, Dam and Onland-Moret54,Reference Staessen, Ginocchio and Thijs55) and lipids(Reference Derby, Crawford and Pasternak56,Reference Wang, Ferreira and Nelson57) , inflammation(Reference Wang, Ferreira and Nelson57,Reference Clayton, Soares and Kilpi58) , central adiposity(Reference Clayton, Soares and Kilpi58,Reference Ambikairajah, Walsh and Tabatabaei-Jafari59) , impaired glucose homeostasis(Reference Clayton, Soares and Kilpi58) and vascular dysfunction(Reference Iwamoto, Sakamoto and Tsuchida60). Arguably, an additional CVD risk factor associated with menopause is very likely to be reduced conversion of ALA to EPA and DHA, potentially leading to increased requirements for dietary EPA and DHA after menopause. Greater dietary LCn-3 PUFA requirements after the menopause may mean that postmenopausal women respond to supplementation more favourably than men, evidence for which will be discussed in the next section.
Evidence supporting the role of LC-n-3 PUFA supplementation for prevention of CVD in women
Decades of research points to the beneficial cardiovascular health effects of consuming n-3 fatty acids(Reference Innes and Calder61). Epidemiological evidence has been pivotal in the development of dietary guidelines for fish consumption for prevention of CHD(39). Few epidemiological studies have focused on women, but a notable example was the Nurses’ Health Study which reported that women consuming fish 5 or more times a week had a 32 % reduced risk of all-cause mortality and 31 % reduced risk of total CHD compared to those consuming less than once a month(Reference Hu62). In a meta-analysis study that showed a dose-response relationship between fish consumption and CHD incidence(Reference Zhang, Xiong and Cai63), a sub-analysis was conducted on 8 of the studies where analysis by sex was possible; this showed that there was a 15 % lower relative risk of CHD incidence in females, but no associations in males for CHD incidence, suggesting that the relationship between fish consumption and CHD incidence may be stronger in women(Reference Zhang, Xiong and Cai63).
Limitations of using prospective cohort studies on fish consumption as evidence for the role of LC n-3 PUFA in disease risk include residual confounding from other elements of the diet that often co-exist with patterns of fish consumption (for example higher intakes of fish may mean lower intakes of red meat), as well as other factors. In addition, variability in n-3 PUFA or marine contaminant content cannot be taken into account, and it is impossible to isolate the contribution of n-3 PUFA from other oily fish-derived nutrient components such as iodine, selenium and vitamin D. Randomised controlled trials (RCTs) are needed to confirm causal effects of LCn-3 PUFA on CHD risk. Many of the RCTs that included CVD events or mortality as primary outcomes have tested pharmaceutical-grade fish oil or purified fatty acids as the intervention(Reference Abdelhamid, Brown and Brainard64). It is well-established that EPA/DHA supplements are particularly effective in tackling hypertriglyceridaemia(Reference Wang, Zhang and Zhou38,Reference Wei and Jacobson65) , which is associated with a greater risk of CHD in women compared to men(Reference Castelli16). FINGEN was a key RCT powered to investigate sex differences; it was reported that 0·7 g/d EPA + DHA reduced triglycerides to a similar extent in men and women, but that the higher dose of 1·8 g/d (roughly equivalent to 4 portions oily fish per week) was more effective in men(Reference Caslake, Miles and Kofler66). More evidence is needed from trials specifically designed to test whether there are female-specific age- or menopause-related changes in lipid responses to fish oil supplementation in order to support life course-targeted advice on fish oil supplementation.
Although many studies such as FINGEN reported effects of LC n-3 PUFA on fasting TAG(Reference Caslake, Miles and Kofler66,Reference Sanders, Hall and Maniou67) , in fact, non-fasting TAG are superior in predicting risk of CHD(Reference Egeland, Igland and Sulo17). We have recently reported that postmenopausal status is associated with higher postprandial triglyceride concentrations following high-fat meals in the predominantly female PREDICT 1 UK cohort (n 1002; pre- n 366, peri- n 55 and postmenopausal females n 206; adjusted for age, BMI, HRT and smoking status)(Reference Bermingham, Linenberg and Hall68), although in analysis of a small age-matched cohort to separate age-related effects from oestrogen status, this was not replicated (pre- n 86 and postmenopausal n 64). However, this does not rule out an important role of oestrogen in the relationship between non-fasting TAG and risk of heart disease. Interestingly, postprandial lipaemia was lower in women on HRT compared with those not on HRT suggesting that oestrogen is likely to have an important role in postprandial lipid metabolism(Reference Bermingham, Linenberg and Hall68). Unfortunately, most chronic fish oil interventions with postprandial lipaemia as an outcome are either in men and or not designed to report sex-specific responses so it is currently unclear whether menopausal status modifies the effects of fish oil on non-fasting triglyceride concentrations(Reference Slivkoff-Clark, James and Mamo69–Reference Harris, Connor and Alam74).
The first large randomised controlled trials of LC n-3 PUFA and CHD outcomes started to emerge in the 1990’s and 2000’s showing promising reductions in secondary prevention of CHD(Reference Yokoyama, Origasa and Matsuzaki75–Reference Burr, Gilbert and Holliday78). However, by the 2010’s, evidence was mounting that n-3 fatty acids were not effective in preventing CHD in either primary prevention (in populations without prior disease) or secondary prevention (in populations with existing disease) populations(Reference Kalstad, Myhre and Laake79–Reference Rauch, Schiele and Schneider84), and there have been many theories on why these trials failed to replicate earlier results(Reference Rice, Bernasconi and Maki85). From 2012 until 2018, a number of meta-analyses concluded there was insufficient evidence for benefits of fish oil supplementation in the prevention of various outcomes including CVD/CHD deaths, cardiovascular events, sudden cardiac death, cardiac events/MI and stroke(Reference Balk, Adam and Langberg86–Reference Kwak, Myung and Lee89), but the inclusion of two later trials in post-2018 meta-analyses resulted in modest but statistically significant pooled risk reductions of 8–10 % in risk for CHD death(Reference Abdelhamid, Brown and Brainard64,Reference Hu, Hu and Manson90,Reference Khan, Lone and Khan91) , total CHD(Reference Hu, Hu and Manson90), CVD death(Reference Abdelhamid, Brown and Brainard64,Reference Hu, Hu and Manson90,Reference Khan, Lone and Khan91) , total CVD(Reference Hu, Hu and Manson90), CHD events(Reference Abdelhamid, Brown and Brainard64,Reference Khan, Lone and Khan91) and total MI(Reference Abdelhamid, Brown and Brainard64,Reference Hu, Hu and Manson90,Reference Khan, Lone and Khan91) . The VITAL (0·85 g/d EPA + DHA ethyl esters)(Reference Manson, Cook and Lee92) and REDUCE-IT (4 g/d icosapent ethyl; 100 %EPA)(Reference Bhatt, Steg and Miller93) trials reported that LCn-3 PUFA supplementation significantly reduced risk of total MI by 28 % and 31 %, respectively, and risk of coronary revascularization (specifically percutaneous coronary intervention in the VITAL study, not including coronary artery bypass grafting) by 22 % and 34 % respectively(Reference Manson, Cook and Lee92,Reference Bhatt, Steg and Miller93) , although the VITAL trial found no statistically significant effect on its primary endpoint, a composite of myocardial infarction, stroke or death from cardiovascular causes(Reference Manson, Cook and Lee92).
In nearly all these RCTs, the proportion of women in the sample population was low (Table 2). Studies that showed no benefit ranged from 10–38 % of participants being female(Reference Kalstad, Myhre and Laake79–Reference Rauch, Schiele and Schneider84,Reference Nicholls, Lincoff and Garcia94,95) , and in those that showed benefit the range was 15–69 %(Reference Yokoyama, Origasa and Matsuzaki75,Reference Marchioli, Barzi and Bomba77,Reference Manson, Cook and Lee92,Reference Bhatt, Steg and Miller93) . The only study that was designed and statistically powered to look at sex differences was the VITAL trial, which included healthy men above the age of 50 and healthy women above the age of 55 years(Reference Manson, Cook and Lee92); no sex differences in risk reductions were shown across the whole sample, although presence or absence of significant interactions with potential modifiers of treatment by sex interactions such as ethnicity, background dietary intake of fish/tissue EPA and DHA status, or BMI were not presented. Based on this single trial, evidence suggests that men and postmenopausal women benefit similarly from EPA and DHA supplementation, at least in primary prevention populations where the prevalence of fish consumption was relatively high (median intake 1·5 servings/week), use of HRT was low (12 %), and the dose of EPA and DHA was equivalent to the amount that can be derived from consuming 2 portions of oily fish per week(Reference Manson, Cook and Lee92). Further evidence is needed to determine whether HRT might improve responsivity to LCn-3 PUFA supplementation in women, particularly among women who consume very little fish.
Table 2. A selection of randomised controlled trials (where n > 500 per randomised group) of LC n-3 PUFA supplementation in the primary and secondary prevention of CVD, showing dose of EPA + DHA, or EPA, the percentage of the sample population that was female, primary outcomes and any analysis by sex

Abbreviations: EPA, eicosapentaenoic acid; DHA, DHA; MI, myocardial infarction; TIA, transient ischaemic attack.
The REDUCE-IT trial, which reported a significant 25 % reduction in major adverse cardiovascular events including a 20 % reduction in cardiovascular death(Reference Bhatt, Steg and Miller93), differed from other LCn-3 PUFA supplementation studies in several study design features. Firstly, like the 2007 JELIS trial(Reference Yokoyama, Origasa and Matsuzaki75), the capsules contained EPA only, but as a purified EPA ethyl ester, and at a very high dose (4 g/d)(Reference Bhatt, Steg and Miller93). All participants were hypertriglyceridaemic, statin-treated CVD patients or had diabetes plus another risk factor. The placebo control capsule contained 4 g/d pharmaceutical-grade mineral oil rather than a culinary oil, and there has been much debate on whether the mineral oil compromised the results by having unfavourable effects on inflammation(Reference Doi, Langsted and Nordestgaard96,Reference Olshansky, Chung and Budoff97) . Nevertheless, evidence from the REDUCE-IT trial was considered strong enough to form the basis of new medical guidelines in many countries(Reference Miller, Tokgozoglu and Parhofer98), which supported 4 g/d icosapent ethyl (pure EPA ethyl esters) treatment for lowering CV risk in patients with high blood TAG concentrations, with established atherosclerotic CVD or diabetes with risk factors, and being treated with statins. Considering the dynamic relationship between changes in oestrogen, CVD risk factors, and tissue LC n-3 PUFA status following menopause, it could be argued that there is strong justification for conducting more research on sex differences in the effectiveness of icosapent ethyl on health outcomes. However, for decades women have faced historical disparities in the management of CVD, with research and clinical practices often being disproportionately weighted towards male patients. This has resulted in underdiagnosis, misdiagnosis and suboptimal treatment for women. Women are less likely to be prescribed lipid-lowering medication in primary care(Reference Jacob, Greiner and Luedde99,Reference Zhao, Woodward and Vaartjes100) or as part of secondary prevention treatment for CVD(Reference Wilkinson, Bebb and Dondo101), to undergo diagnostic procedures for atherosclerosis(Reference Wilkinson, Bebb and Dondo101), or to be referred to, or able to access, cardiac rehabilitation(102,Reference Li, Fonarow and Mukamal103) ; time will tell whether prescription and evaluation of high-dose EPA for CVD prevention will be equitable across sexes.
Causes of differential CVD risks throughout the female life course
LCn-3 PUFA supplementation primary and secondary prevention studies tend to focus on populations at midlife and older due to statistical power constraints. However, the involvement of n-3 fatty acids in the maintenance of cardiovascular health is not confined to later life. Modifiable CVD risk factors, present from childhood and early adolescence onwards(Reference McGill, McMahan and Herderick104), have consequences for atherogenesis and cardiovascular health throughout the whole life course. In fact, there are sex-specific factors at many stages of a woman’s life that may interact with diet to impact the rate of CVD risk accrual (Fig. 1).

Fig. 1. Female-specific cardiovascular risk factors through the life course.
Menstrual and ovarian abnormalities
Menstrual abnormalities are associated with chronic inflammation and hormonal irregularities, and aspects of menstrual abnormalities such as irregular cycles(Reference Okoth, Smith and Thomas105,Reference Solomon, Hu and Dunaif106) , pre-menstrual tension(Reference Bertone-Johnson, Whitcomb and Rich-Edwards107), menorrhagia(Reference Chung, Ferreira and Mishra108) and dysmenorrhoea(Reference Yeh, Muo and Sung109) have been associated with increased risks of CHD or hypertension. Fish oil supplements may have some beneficial impact on the severity of these conditions but studies are small and heterogeneous so more evidence is needed(Reference Mohammadi, Dehghan Nayeri and Mashhadi110,Reference Snipe, Brelis and Kappas111) . Polycystic ovary syndrome (PCOS) is a hyperandrogenic endocrine disorder that begins during puberty and causes polycystic ovarian morphology, ovulation disorders, irregular periods, hirsutism and acne. Insulin resistance is present in 65–80 % of women with PCOS(Reference Zhao, Zhang and Cheng112), as well as a greater prevalence of CVD risk factors(Reference Ollila, Piltonen and Puukka113,Reference Ollila, Kaikkonen and Järvelin114) . LCn-3 PUFA supplementation may be beneficial in reducing CVD risk factors in women with PCOS, but there is currently a lack of convincing interventional evidence to make recommendations(Reference Xia, Wang and Cui115). The British Dietetic Association, however, recommends dietary omega-3 FA in women living with PCOS.
Adverse pregnancy outcomes
Many studies have shown that various pregnancy complications are associated with an increased risk of CHD(Reference Crump, Sundquist and McLaughlin116,Reference Parikh, Gonzalez and Anderson117) . The presence of underlying risk factors for CVD, such as a predisposition to high blood pressure or diabetes, combined with the physiological stress of carrying and growing a foetus, may result in adverse pregnancy outcomes. On top of this, enduring and surviving a pregnancy complication may cause long-term damage that may render the women more susceptible to developing CVD in later life. The relative risk of CHD events associated with pregnancy complications is estimated at 1·49 (1·38–1·60) for preterm delivery(Reference Wu, Gulati and Kwok118), 2·50 (1·43–4·37) for pre-eclampsia(Reference Wu, Haththotuwa and Kwok119) and 1·59 (1·30–1·94) for gestational diabetes(Reference Li, Song and Li120). Pregnancy complications have also been associated with increased risk of hypertension, diabetes and dyslipidaemia in later life which may mediate the increased risk of CVD(Reference Parikh, Gonzalez and Anderson117).
Tissue LCn-3 PUFA metabolism could be a significant factor in the pathology of adverse pregnancy outcomes. Maternal and cord blood DPA and DHA concentrations in women with pre-eclampsia were 55–67 % of control maternal and cord blood levels and similar patterns were observed in women with intrauterine growth restriction(Reference Mackay, Huda and Stewart121). Maternal EPA was slightly higher in women with pre-eclampsia (∼121 %) than control maternal EPA concentrations(Reference Mackay, Huda and Stewart121), suggesting that this may be at least in part attributable to reduced conversion of EPA to DHA. Further investigation revealed reduced subcutaneous (but not visceral) adipose tissue FADS1 and FADS2 gene expression and an absence of elongase expression in samples from women with pre-eclampsia, lending further support to the notion that this condition is linked to the disruption of EPA conversion to DHA(Reference Mackay, Huda and Stewart121). However, it is unclear whether the disrupted EPA to DHA conversion is in the causal pathway for pre-eclampsia, or whether the pathology of pre-eclampsia and disruption to conversion enzymes both arise from a common origin.
Supplementation intervention trials could shed light on whether low conversion rates are causal in the pathology of pregnancy complications. A meta-analysis of RCTs concluded that supplementation may reduce risk of pre-eclampsia but it is uncertain, with a calculated overall relative risk that was not statistically significant (RR 0·84, 0·69–1·01; 20 trials, n 8306), plus the evidence was rated low quality(Reference Middleton, Gomersall and Gould122). However, the evidence strongly suggests that LCn-3 PUFA supplementation during pregnancy reduces risk of preterm birth by 11 % (RR 0·89, (0·81–0·97); 26 trials, n 10 304; high-quality evidence), with an even stronger reduced risk of early (<34 weeks) preterm birth (RR 0·58, (0·44–0·77) trials, n 5204; high-quality evidence); indeed there was a greater incidence of very long pregnancies (>42 weeks) following supplementation(Reference Middleton, Gomersall and Gould122).
Menopause
The decline in oestrogen production has significant implications for body tissue LCn-3 PUFA status, as discussed at an earlier point in this review. However, simply replacing oestrogen through hormone replacement therapy may not be suitable for all postmenopausal women. Oral oestrogen plus progestin therapy was found to increase CHD risk during the first year of treatment in women in late menopause in the Heart and Estrogen/progestin Replacement Study (women with CHD) and Women’s Health Initiative (WHI) studies (some women with but most without CHD)(123,Reference Hulley124) , and there was an increasing risk of CHD with increasing time since menopause(Reference Rossouw, Prentice and Manson125). However, in postmenopausal women who were less than 10 years post-menopause, taking oral oestrogen or oestrogen plus progestin reduced risk of CHD by 24 %(Reference Rossouw, Prentice and Manson125) and risk of all-cause mortality by 30 %(Reference Boardman, Hartley and Eisinga126), although there was still a 74 % increased risk of venous embolism with oral HRT(Reference Boardman, Hartley and Eisinga126). A subset of women taking part in the WHI RCT on oral hormone replacement therapy (consuming oestrogen alone or oestrogen plus progestin) had a 10 % reduction in DPA and 11 % increase in DHA in erythrocyte membrane lipids compared to the control group after 1 year of therapy(Reference Harris, Tintle and Manson52), and similar findings were demonstrated in healthy, early postmenopausal women taking combined oral oestrogen and progestin(Reference Giltay, Duschek and Katan127). In addition, a small study reported that women receiving DHA supplementation had lower increases in EPA and higher DHA:EPA ratios if they were taking oral HRT compared to those not on HRT, suggesting reduced retroconversion of DHA to EPA(Reference Stark and Holub128). Increasingly, peri- and postmenopausal women are now being prescribed transdermal oestrogen (rather than oral)(Reference Alsugeir, Wei and Adesuyan129), due to its efficacy in resolving menopausal symptoms and a superior safety profile. Transdermal oestrogen treatments are applied at much lower doses and are metabolised differently to oral treatments, avoiding first-pass hepatic metabolism to pass directly into the bloodstream. Although there is no evidence to suggest that oral and transdermal oestrogen have a differential impact on risk of CVD(Reference Goldštajn, Mikuš and Ferrari130,Reference de Vries, Bromley and Farmer131) , it is unlikely that transdermal oestrogen reaches the liver in a high enough dose to modulate PPAR activity thus probably having little effect on PUFA metabolism.
Inflammatory burden increases during menopause(Reference Bermingham, Linenberg and Hall68) due to the declining impact of oestrogen on optimal function of immune cells(Reference Gameiro, Romão and Castelo-Branco132), skin and mucosal integrity(Reference Rahn, Good and Roshanravan133), musculo-skeletal function(Reference Watt134), moderating cytokine responses to injury/stress(Reference Gameiro, Romão and Castelo-Branco132) and resolving inflammatory events(Reference Villa, Rizzi and Vegeto135). The role of EPA and DHA in dampening chronic low-grade inflammation may be especially important during this stage of a woman’s life, particularly as the majority of postmenopausal women consume little or no oily fish (Table 1).
The distinct contribution of the range of traditional and sex-specific risk factors to total risk of CVD may differentially modulate the cardioprotective effects of LCn-3 PUFA intake by biological sex, yet dietary guidelines currently do not fully take account of life stages where requirements may be higher in females.
Conclusions
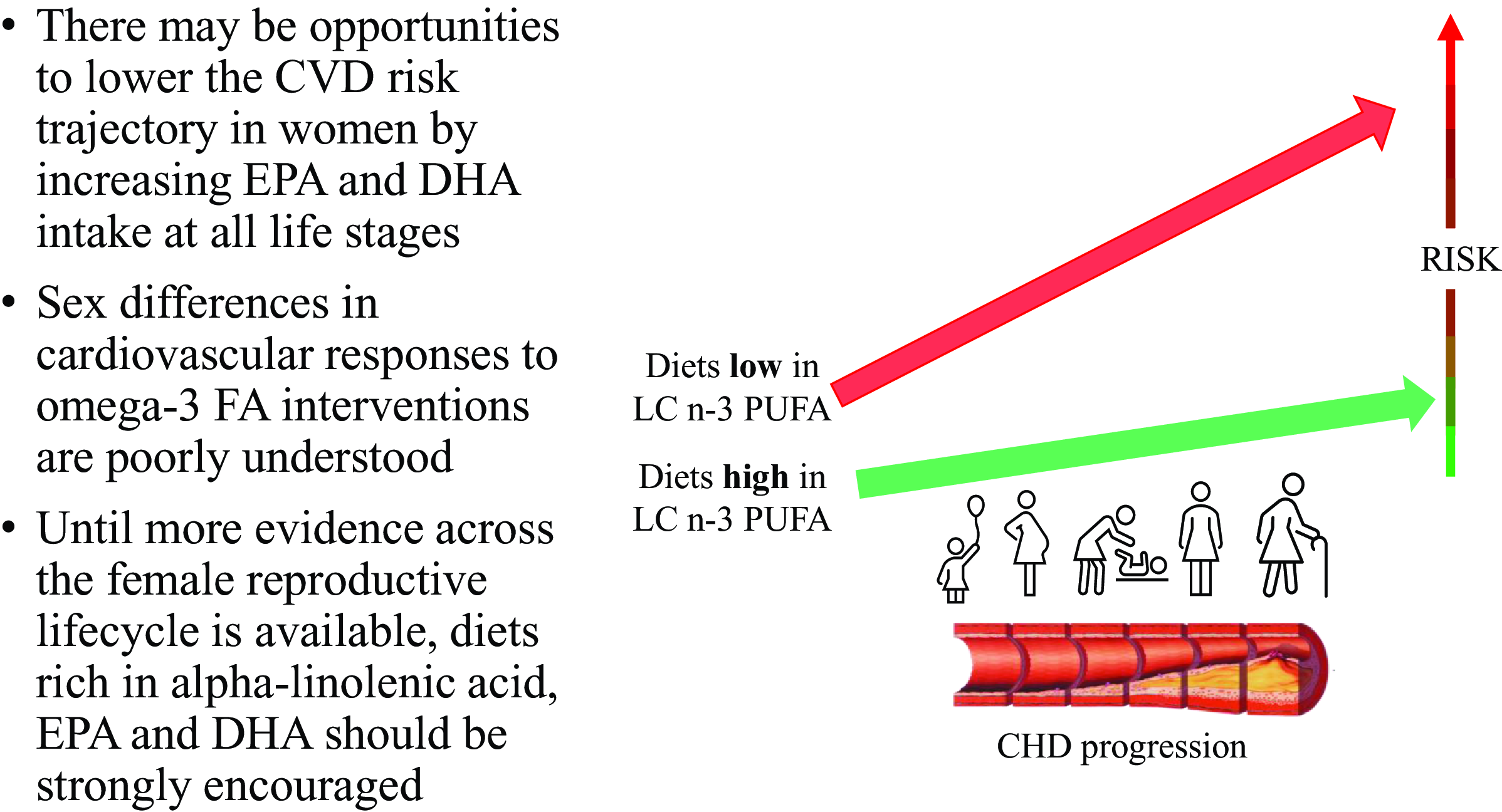
Many women may have additional female-specific CVD risk factors and greater vulnerabilities to traditional risk factors that arguably put oily fish dietary guidelines as a higher priority for prevention of cardiac death and morbidity compared with men. The existence and magnitude of sex differences in responses to LCn-3 PUFA interventions are poorly understood due to sex comparisons not being built into study designs a priori and lack of consideration of background oestrogen levels. There is an urgent need for more research on cardioprotective dietary interventions across the reproductive lifecycle of women, particularly dietary/supplementary sources of LCn-3 PUFA. In the meantime, while female-specific evidence is lacking, consumption of oily fish or alternative non-fish sources of LCn-3 PUFA should be strongly encouraged as part of dietary recommendations for women.
Acknowledgements
W.H. would like to thank the Organising Committee of the Nutrition Society Summer Conference 2023 for the speaker invitation.
Financial support
This work did not receive a specific grant from any funding agency, commercial or not-for-profit sectors.
Author contributions
WH wrote the manuscript and is responsible for the final content.
Competing interests
There are no conflicts of interest to declare.