Introduction
The immense public popularity of theropod dinosaurs has generated considerable marketing of bone and bone-replica models, books, clothing, artwork, toys, novelties, theatrical presentations, and traveling animatronic dinosaur model shows. Those who study theropods often acknowledge this popularity in their publications [Reference Chinsamy1–Reference Bouabdellah8], and, as one worker has put it, “Nearly any five-year-old in the industrialized world knows what it is, and to many, Tyrannosaurus is the quintessential predatory dinosaur … T. rex is as common in the popular media as Tyrannosaurus.” [Reference Brochu9].
Histology of theropod dinosaur bones and the vascular canals within them harks back to the turn of the 20th century when museums openly, and sometimes fiercely, competed to establish high-quality bone collections and prep them for study and/or display [Reference Colquhoun10–Reference Brinkman and Brown12]. Since then, histological studies of theropod bones, particularly tyrannosauroid bones, have, with great advances in histological methods and technology, advanced our understanding of the relationship between osteomorphology and dinosaur dynamics [Reference Woodward7, Reference Horner13–Reference Cullen22]. One controversial theropod dinosaur, Nanotyrannus lancensis [Reference Bakker and Williams24], has been the subject of a tyrannosauroid phylogeny debate for decades. Recently, specimens of N. lancensis were sectioned and histological results offered as refutation of the disputed taxon [Reference Woodward7]. Many argue that it has characteristics which make it deserving of it own taxonomic status [Reference Gilmore23–Reference Schmerge and Rothschild30]. Others are convinced that N. lancensis merely represents a juvenile T. rex [Reference Woodward7,Reference Carr31–Reference Brusatte and Carr35]. Either way, histology of N. lancensis (or T. rex) isolated post-cranial bone elements collected in 2021 at the Hell Creek Formation (Jordan, MT) is the subject of the present study. We present new UV fluorescence microscopy histological data revealing the presence of blood clots in this controversial tyrannosauroid.
Materials and Methods
Nanotyrannus lancensis specimens were collected from sandy escarpments within the Hell Creek Formation, Jordan, MT, in 2021. Post-cranial isolated (and in many cases fractured) elements including radius, ulna, and phalanx were collected and immersed in 10% phosphate buffered formalin. An isolated N. lancensis tooth and claw were also recovered. Specimens were washed, air-dried, and 40-micron ground sections prepared. Non-coverslipped sections were viewed with 347 nm UV autofluorescence microscopy for evidence of blood clots in vascular canals.
Results
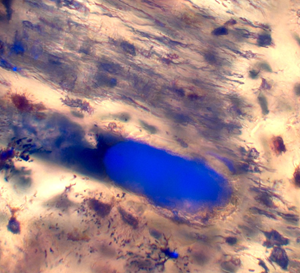
Ground sections of six post-cranial, isolated elements of N. lancensis (radius, Figure 1 A–D; digit, Figure 2 A–C; limb element, Figure 3 A–C; vertebra, Figure 4 A–C; ulna, Figure 5 A–C; and claw, Figure 6 A–C) were examined with 347 nm UVFL microscopy. All were positive for iron clots within canals. One double serrated cranial N. lancensis tooth (Figure 7 A–E) was negative for clots, however, dental enamel was partially intact (Figure 7C).

Figure 1: Specimen DSTRI831H N. lancensis. (A) Post-cranial, isolated, and fractured radius element sent for sectioning. (B) Brightfield image of a clot occluding a vascular canal in the radius. Scale bar = 40 μm. (C) Brightfield/UVFL image of a clot occluding the vascular canal in the radius. Scale bar = 40 μm. (D) Brightfield/UVFL image of clots filling vascular canals in the radius. Scale bar = 70 μm.

Figure 2: Specimen DSTRI831N N. lancensis. (A) Post-cranial, isolated digit element sent for sectioning. (B) Brightfield/UVFL image of a clot filling the vascular canal in a post-cranial digit element. Scale bar = 25 μm. (C) Brightfield/UVFL image of a clot filling the vascular canal in a post-cranial digit element. Scale bar = 25 μm.

Figure 3: Specimen DSTRI91B N. lancensis. (A) Post-cranial, isolated, and fractured limb element sent for sectioning. (B) Brightfield/UVFL image of a clot filling the vascular canal in a post-cranial limb element. Scale bar = 10 μm. (C) Brightfield/UVFL image of a clot filling the vascular canal in a post-cranial limb element. Scale bar = 10 μm.

Figure 4: Specimen DSTRI91C N. lancensis. (A) Post-cranial isolated vertebra element sent for sectioning. (B) Brightfield/UVFL image of a clot filling the vascular canal in a post-cranial vertebra element 4. Scale bar = 100 μm. (C) Brightfield/UVFL image of a clot filling the vascular canal in a post-cranial vertebra element. Scale bar = 100 μm.

Figure 5: Specimen DSTRI91F N. lancensis. (A) Post-cranial, isolated, and fractured ulna sent for sectioning. (B) Brightfield/UVFL image of clots filling vascular canals in a post-cranial ulna element. Scale bar = 30 μm. (C) Brightfield/UVFL image of clots filling vascular canals in a post-cranial ulna element. Scale bar = 30 μm.

Figure 6: Specimen DSTRI91H N. lancensis. (A) Post-cranial, isolated claw. (B) Brightfield/UVFL image of clots filling vascular canals in a post-cranial claw element. Scale bar = 30 μm. (C) Brightfield/UVFL image of clots filling vascular canals in a post-cranial claw element. Scale bar = 30 μm.

Figure 7: Specimen DSTRI831Mz N. lancensis. (A) Isolated, serrated tooth, sent for sectioning. No clots were found. (B) Isolated, serrated tooth, from Figure 7A, showing convex serrations (arrows). (C) Polarized light image using crossed polars with lambda plate, ground section of tooth from Figure 7A showing enamel layer. Scale bar = 120 μm. (D) Brightfield image of tooth from Figure 7A showing inner serrations. Scale bar = 1 mm. (E) Scanning electron microscope image of tooth from Figure 7A showing inner serrations.
Classically shaped round canals and long thin canals surrounded by whorls of osteocytes in both haversian and fibrolamellar bone characterized vascular canals (Figures 1 B–C, 2 B–C, 3 B–C, 4 B–C, 5 B–C, and 6 B–C). Clots fluoresced brightly, exhibited fine structure or particulate material within them, and bore malleable polishing marks, except for the clots in the digit (Figures 2 B–C), which showed no clear polishing marks or structure and particulate material in the clots. This may have been the result of incomplete dehydration before examination in UVFL, rendering the auto-fluorescent signal diffuse.
In addition to clots, roundworms or nematodes were observed in canals (Figures 4B and 8). Other reports may have also observed nematodes in dinosaur bones but may have misidentified these due to processing and imaging differences between the studies.

Figure 8: Specimen HCTH-00. Polarized light image of ground section of Triceratops horn showing a probable nematode preserved in a vessel canal using crossed polars with lambda plate. Scale bar = 45 μm.
Discussion
Morphological bone data obtained from ground sections affords an understanding of the degree of vascularization in dinosaur remains. This attribute has direct relation to dinosaur physiology, life cycle, growth rate, behavior, feeding specializations, sensory capability, disease, parasitism, and predation [Reference Chinsamy1,Reference Clarey3,Reference Woodward7,Reference Colquhoun10,Reference Horner13–Reference Brink20,Reference Cullen22,Reference Aureliano36]. Bone morphology can also be used to estimate the actual age of the dinosaur at death [Reference Woodward7,Reference Horner and Padian14].
Many rare dinosaur bone specimens are discovered crushed, fractured, damaged by predators, weathered, or accompanied by artifacts of taphonomic processes [Reference Currie2,Reference Yun5,Reference Horner13,Reference Schweitzer17,Reference Brochu37]. This is especially true of skulls [Reference Clarey3,Reference Brochu9,Reference Gilmore23,Reference Schmerge and Rothschild29,Reference Schmerge and Rothschild30,Reference Carr31,Reference Witmer and Ridgely32,Reference Brochu37–Reference Witmer, Endo and Frey39]. Thus, the quality and anatomic accuracy of neurovascular canal images from such specimens can be adversely impacted. In some cases, only a few or even one specimen of a given taxon exists. Additionally, sectioning of bone (especially serial sectioning) is a destructive technique and may be cost-prohibitive depending on the size and shape of the bone. Moreover, workers are simply reluctant to subject rare or exceptionally well-preserved specimens to such a process. Other problems exist, like the difficulty in rendering 3-D images from serial ground sections, and the fact that the entire bone is made unavailable for further study after serial sectioning. Further, collection, processing, and sectioning methods can introduce artifacts, rendering images that cannot be interpreted.
In recent years, a non-destructive computed tomography (CT) scanning of dinosaur bones has also been employed, resulting in remarkable images of neurovascular and other canals [Reference King6,Reference Bouabdellah8–Reference Brochu9,Reference Brochu37–Reference Kawabe and Hattori43]. This is especially notable with respect to imaging dinosaur jaws (maxillae and mandibles) [Reference Bouabdellah8,Reference Bell40,Reference Kawabe and Hattori43]. Soil matrix and metal artifacts can be identified by CT scanning [Reference Brochu37], however none of the referenced dinosaur bone CT studies have reported blood products in neurovascular canals. Soft neurovascular elements are not visualized in isolation, and only the cranial cavities are imaged in digital endocasts [Reference Cerroni42].
Bone has a large mineral component yet is amply supplied by soft tissue neurovascular bundles [Reference Bouabdellah8,Reference Sakagami and Kawabe42–Reference Kawabe and Hattori43]. It would be expected, therefore, that a rich supply of blood vessels, veins, and nerves would have been present in dinosaur bones at death. A post-mortem condition known as livor mortis occurs when gravity-created blood pools form at the lowest part of a decumbent body ([Reference Schweitzer17] pp. 64–65). Blood pooling within dinosaur bones might have occurred as well but was not studied.
Reports of blood in histological sections or decalcified soft material from dinosaur bones are rare [17,44 (Fig. 16),45]. One worker reported observations of “small red spheres … which lay in a blood vessel channel that wound through the pale, yellow hard tissue,” [Reference Schweitzer17]. Moreover, the report noted, “Hollow, transparent, flexible branching tubes … and they looked exactly like blood vessels. Suspended inside the vessels were either small, round red structures or amorphous accumulations of red material” [Reference Schweitzer17]. We interpret these “amorphous accumulations of red material” to be the remains of blood clots within the original dinosaur blood vessels and canals.
Other clots [36, pp. 5–7] compel us to comment on putative vascular channel blood parasites detailed recently in an isolated Brazilian sauropod dinosaur fibula. The authors describe these fusiform-bodied putative vascular parasites as “indeterminate fossilized microorganisms” and “soft-bodied parasitical microorganisms.” Histological sections show that the fibula was deeply infiltrated by calcite (and possibly sediment), as indicated by polarization microscopy [36,Figures 5A–B,D,F–H]. Researchers described this as “moderate infilling of mineral grains.” However, the calcite infiltration was widespread. The fusiform shapes, only observed within vessel canals, are reported to have a constant morphology, that is, “always showing one subtle more tapered end.” Also observed were internal “dark/more opaque spots” within the putative parasite.
It appears the Titanosaur fibula was not fixed prior to resin infiltration [Reference Aureliano36]. We routinely fix, wash, and dehydrate samples prior to embedding in hopes of preserving in detail any helminth or microbial communities within bone and to preserve the integrity of clots remaining in canals. The clots that we present here and elsewhere [Reference Armitage and Anderson44,Reference Armitage and Solliday45], in addition to clots we discovered upon UVFL examination of Camarasaurus post-cranial elements (unpublished), are generally thicker, more uniform in their close adherence to canal walls, and more responsive to UVFL microscopy, precisely because of our methodology.
The dark, opaque, and otherwise featureless nature of the fusiform shapes in the Titanosaur fibula is due to lack of fixation. Post-burial infiltration of calcite into the fibula preserved some details of the shapes, but most structural detail is lost. In contrast, a canal clot in N. lancensis (Figure 4B) features a fusiform body of a similar shape and size with clear internal structures. We interpret this fusiform microorganism in the N. lancensis vascular canal, and that shown in the horn (Figure 8), to be nematodes or other roundworms preserved by fixation. Moreover, we maintain that the Titanosaur microorganisms have been misidentified as blood parasites, which are known to be much smaller than roundworms [36, p. 10]. We have observed nematodes routinely in ground sections of our freshly-excavated-and-fixed specimens from as early as 2012, (Triceratops horridus horn, HCTH-00) [44, Figures 12,16]. Nematodes have only been observed in canals.
Interestingly, the nematode from the horn has the same “dark/more opaque spots with a larger one located centrally and a smaller one located close to the more tapered end” as described in the Titanosaur fibula [36, p. 8], but other internal details, including possible organelles, are lacking in the fibula worm. In contrast, Figure 8 shows “dark/more opaque spots” with an identical location and description as the Titanosaur finding. We maintain that preservative fixed the bodies and internal organelles of living nematodes within the Nanotyrannus and Triceratops specimens, yielding the superior preservation and detail seen here. It should not be surprising to find nematodes, given the large numbers of microbes that have been found inhabiting dinosaur bones [Reference Liang46].
The brown material immediately and closely adhering to vessel canal walls was not discussed in [Reference Aureliano36]. We interpret that brown material (more expansive in Figure 5D [Reference Aureliano36]) to be clotted blood from the Titanosaur fibula (which was probably fractured during sediment re-working, similar to the elements in this report). Significantly, we have consistently observed these clots in our dinosaur bone section preparations. They always fluoresce with UV microscopy using 347 nm illumination. Moreover, we interpret many published dinosaur bone sections to contain similar clots [36,Reference Klein and Sander49–Reference Skutschas52]. One report stated, “Vascular canals are in-filled with a dark ferric material” [47, p. 557]. It is likely that the ferric material is the remains of blood clots in the canals. Furthermore, we note that none of these theropod histological reports indicate the use of a fixative in methodology. The clots that we observe in formalin-fixed specimens are thicker, more prevalent, and more resistant to dislocation during processing than are unfixed bones.
Another researcher [53] working with fossil bones of freshwater Metoposaurs, showed multiple bone thin sections with canals filled with sediment, calcite, and pyrite. They acknowledge the presence of iron and sulfate-reducing bacteria acting on soft tissues and iron, but then they attribute infiltration of pyrite into canals to negative pressure within the bone as a result of bacterial outgassing. We wonder why original blood and blood vessels would not suffice as the source of iron and sulfates from bacterial-induced decomposition of tissues already present in vascular canals upon death.
Conclusions
UVFL microscopy of vascular canals within Cretaceous theropod bone specimens shown here reveals the presence of blood-iron clots, trapped in canals during a probable asphyxiation/drowning event for this animal. This provides further proof of probable disseminated intravascular coagulation and death by drowning among theropod dinosaurs similar to previous reports of clots in sauropod specimens (Triceratops) [Reference Armitage and Anderson44,Reference Armitage and Solliday45]. We also re-interpret the crystallized structures within the lumen of the blood vessel [44, Figure 16, p. 607] to be clotted blood adhering to the vessel lumen wall.
UV autofluorescing soft-tissue clots have been found in Cretaceous sauropods (Triceratops and Camarasaurus) and now theropods (Nanotyrannus). This extends the range and type of dinosaurs that suffered a catastrophic thrombus event while drowning. Fixation in aldehyde prior to embedment appears to provide superior preservation of clots and other microscopic structural detail, including possible nematode infestation. Several published reports employing ground sections appear to show clots in vessel canals, as well as nematodes residing in clotted canals. Renewed examination of existing theropod ground sections might reveal the unrecognized presence of these clots and nematodes. Workers should subject their existing sections to UVFL microscopy for corroboration of clots and nematodes in dinosaur bones.