Introduction
Cantor's giant softshell turtle Pelochelys cantorii (Gray 1864) is a Critically Endangered yet widely distributed freshwater turtle in South and Southeast Asia (Bour, Reference Bour, Balian, Lévêque, Segers and Martens2008; Das, Reference Das2008; Choudhury et al., Reference Choudhury, Das, Horne, Li, McCormack and Praschag2021). The species belongs to the family Trionychidae, which comprises highly aquatic and predominantly carnivorous turtles (Praschag et al., Reference Praschag, Hundsdörfer, Reza and Fritz2007), with a wide variation in size amongst species (Fitch & Plummer, Reference Fitch and Plummer1975). Being mainly scavengers, softshell turtles are important for freshwater ecosystems because they help maintain water quality and trophic webs (Fitch & Plummer, Reference Fitch and Plummer1975; Ali et al., Reference Ali, Javid, Hussain and Bukhari2018; Lovich et al., Reference Lovich, Ennen, Agha and Gibbons2018). Natural predation of P. cantorii individuals and eggs is not documented, and adult individuals can become apex consumers with no natural predators (de Miranda, Reference De Miranda2017). However, jackals, wild boars, monitor lizards and feral dogs have been observed to predate the eggs and juveniles of other large softshell turtles (Palot & Radhakrishnan, Reference Palot and Radhakrishnan2004).
Pelochelys cantorii is an Evolutionarily Distinct and Globally Endangered (EDGE) species, meaning it is a global priority for conservation (Gumbs et al., Reference Gumbs, Gray, Böhm, Hoffmann, Grenyer and Jetz2020) because of its disproportionate contribution to phylogenetic diversity and high risk of extinction according to the IUCN Red List. It is also listed in Appendix II of CITES, limiting its trade. The species is legally protected in many countries, including Viet Nam, Myanmar, the Philippines and China. In India, the species is listed under Schedule I of the Indian Wildlife (Protection) Act, 1972. Hunting or trade of species listed in Schedule I is an offence punishable with penalties and/or imprisonment. However, management of freshwater fauna, especially outside protected areas, is insufficient in India (Raghavan et al., Reference Raghavan, Das, Nameer, Bijukumar and Dahanukar2016). Law enforcement is generally weak outside protected areas because of limited availability of resources (Aiyadurai et al., Reference Aiyadurai, Singh and Milner-Gulland2010; Milda et al., Reference Milda, Ramesh, Kalle, Gayathri and Thanikodi2020). Additionally, population management and the planning and implementation of conservation interventions for P. cantorii are hampered because data on the species’ ecology, behaviour, population size, distribution and threats are limited.
Most of our knowledge about the ecology and biology of P. cantorii has been obtained from captive populations in China (Chen et al., Reference Chen, Zhou, Peng, Huang and Chen2013; Xinping et al., Reference Xinping, Xiaoyou, Jian, Jianhua and Zicheng2015; Zhang et al., Reference Zhang, Li, Zhao, Chen and Zhu2016, Reference Zhang, Li, Zhao, Chen and Zhu2018; Xiaoyou et al., Reference Xiaoyou, Xiaodan, Chen, Xiaoli, Jian, Quanbo and Xinping2019). Information gathered from the few existing records of the species in the wild suggests that it prefers aquatic habitats, including lakes, rivers, estuaries and coastal areas (Das, Reference Das2008). In India, only 15 records of P. cantorii have been published since 1970 (Jain et al., Reference Jain, Cavada-Blanco, Palot, Das, Deepak and Das2021), many of which are anecdotal or refer to bycatch. Only a few surveys of the species have been carried out, and these failed to document its presence (Sirsi, Reference Sirsi2010; Behera et al., Reference Behera, Panda, Dutta and Nayak2019). Although these studies could suggest local extirpation, the lack of records may also be a result of the turtle's elusive behaviour that reduces detection probability when using traditional ecological survey methods.
Cantor's giant softshell turtle is a highly aquatic species that spends extended periods buried in the sand at the bottom of water bodies (Behera et al., Reference Behera, Panda, Dutta and Nayak2019; Xiaoyou et al., Reference Xiaoyou, Xiaodan, Chen, Xiaoli, Jian, Quanbo and Xinping2019), rendering baited traps ineffective for detecting these individuals (Sirsi, Reference Sirsi2010). Similarly, detection probabilities using visual-based survey methods tend to be low unless previous knowledge suggests the species occurs in the surveyed area.
Because of these difficulties, gathering baseline information about P. cantorii remains challenging, and making even basic plans for the conservation and management of wild P. cantorii populations is problematic at local, provincial and national levels. A possible solution is to utilize local ecological knowledge to gather crucial information on species status, occurrence records and extinction risk (Pan et al., Reference Pan, Wei, Cunningham, Li, Chen, Milner-Gulland and Turvey2016). Local ecological knowledge accumulated by people over their lifetime has proven to be a useful tool for obtaining ecological information on globally threatened, rare and/or elusive species that cannot otherwise be acquired using traditional ecological surveys (Azzurro et al., Reference Azzurro, Moschella and Maynou2011; Turvey et al., Reference Turvey, Fernández-Secades, Nuñez-Miño, Hart, Martinez, Brocca and Young2014; Kanagavel et al., Reference Kanagavel, Parvathy, Tapley, Nirmal, Selvaraj and Raghavan2020). This is a cost-effective approach that is particularly useful for obtaining data on easily identifiable, large and morphologically distinct species (Turvey et al., Reference Turvey, Fernández-Secades, Nuñez-Miño, Hart, Martinez, Brocca and Young2014).
To support the conservation of P. cantorii, we aimed to gather local knowledge on the species’ presence and ecology in Chandragiri River, Kerala, India. We used interview-based surveys to systematically record past sightings, information on hunting and data on the nesting ecology of the species. Through these interviews we also identified key informants and created an active network for the long-term local reporting and management of the species.
Study area
We conducted the study over a 63-km stretch of Chandragiri River in the Kasaragod district of Kerala, India. The Chandragiri River originates in the neighbouring state of Karnataka and flows west into Kerala for c. 110 km, with a total drainage basin of 957 km2 (Chandramohan & Balchand, Reference Chandramohan and Balchand2007). The river supports permanent and temporary freshwater habitats, including streams, marshes and pools, before draining into the Arabian Sea, forming a tropical estuarine system supporting mangrove wetlands. Kerala experiences two monsoon seasons: the south-west monsoon during June–September and the north-east monsoon during October–November. The study area receives mean annual rainfall of 3,350 mm, most of which falls during the south-west monsoon (Diju & Thamban, Reference Diju and Thamban2006). Temperatures are lower in the winter, during November–mid February, with a dry and warm season following that lasts until May.
Kasaragod has 38 political divisions, called grama panchayats, which are further divided into 663 wards. A pilot study in four wards adjacent to Chandragiri River suggested that local knowledge of P. cantorii was influenced by whether people used the river for irrigation and/or subsistence fishing. As the communities residing close to the river tend to visit it frequently, we only targeted 40 wards adjacent to the river in six panchayats: Chemnad, Chengala, Muliyar, Bedadka, Karadka and Delampady (Fig 1). These panchayats jointly had a population of 1,851 people (952 men/boys and 899 women/girls) above the age of 6 years (District Census Handbook, 2011). In this area people predominantly follow Hindu (56%), Muslim (37%) or Christian (7%) religions, with smaller numbers of Sikhs, Buddhists and Jains.

Fig. 1 Study area in Kasaragod district, Kerala, India, including the six panchayats along Chandragiri River where we conducted interview surveys to determine the historical and current presence of Cantor's giant softshell turtle Pelochelys cantorii.
Amongst the communities in the study area, Scheduled Castes and Scheduled Tribes (according to Articles 341, 342, 366(24) and 366(25) of the Indian Constitution; Supplementary Material 1), comprise only 0.04% and 0.02% of the district population, respectively. The most frequently spoken language in Kasaragod is Malayalam, with a smaller number of people also speaking Tulu, Kannada, Konkani and Marathi (District Census Handbook, 2011).
Methods
Data collection
We collected data during June–December 2019 through semi-structured interviews. We initially identified interviewees using chain referral (snowball) sampling, followed by opportunistic sampling (Koerber & McMichael, Reference Koerber and McMichael2008). We asked each interviewed respondent to recommend potential additional interviewees who were knowledgeable about P. cantorii. Most respondents just provided contact details of the referred individual(s), but on occasion the respondent accompanied us for the interview with the referred person. Once the snowball sampling approach provided no further interviewees, we employed opportunistic sampling to improve the representation of our sample. We identified people who lived close to Chandragiri River and were willing to be interviewed. We only interviewed one person per household, unless the family members had different information on turtle sightings. On approaching potential interviewees, we introduced ourselves and described the objectives and purpose of the project and the processes for data management. We then obtained free, prior and informed verbal consent to participate in the study. All interviewees were at least 18 years of age, and they could terminate the interview at any time. We did not share respondent names and personal information with anyone outside the research team. We conducted the interviews in Malayalam, with assistance from a local resident who acted as interpreter. One female and one male interviewer conducted interviews to reduce potential gender biases, and we carried out the interviews individually to avoid social desirability bias (Grimm, Reference Grimm, Sheth and Malhotra2010) and to increase the likelihood of people sharing potentially sensitive information (e.g. on turtle hunting). Some respondents were willing to engage in longer conversations beyond the interviews, providing richer contextual information; we subsequently refer to them as informants.
Interviews comprised 14 items, including open- and close-ended questions. We designed the interviews to: document sightings of P. cantorii (both recent and historical) that we later verified whenever possible; document how local communities use the river; identify the seasonality of turtle sightings, bycatch and nesting activities; and establish trusting relationships on which to build an active monitoring network with local communities.
We asked respondents to describe the appearance of P. cantorii (we used the vernacular name paala poovan as a prompt) and showed them pictures of turtle species including P. cantorii and various native and non-native softshell turtles (Nilssonia leithii, Chitra indica, Lissemys punctata, Apalone ferox), a sea turtle (Lepidochelys olivacea) and a hardshell turtle (Melanochelys trijuga) for validation. In the event of erroneous identification by the respondent, we did not consider the interview data for further analysis. Whenever possible, we verified sightings by asking the respondents to provide photographic proof, visiting the sighting locations and/or corroborating the sighting through supporting interviews. Before concluding the interviews, we encouraged respondents to provide any other pertinent information about turtles that may not have been covered during the interview.
To create an alert network and systematically document any future sightings, we asked all informants to contact a project team member in case of a P. cantorii sighting. Informants who agreed to be a part of the alert network are subsequently referred to as key informants. We contacted key informants frequently to update them on the progress of the project and to conduct informal discussions to obtain any new information on the species, including reports on bycatch, sightings, nesting or hunting. As a part of the alert network, we also asked key informants to invite other interested community members to join the network, irrespective of whether or not they had previously sighted the species. In cases of bycatch or nesting reports, a team of two or three people visited the site to rescue and release the turtle and/or safeguard the nests. We recorded all sightings and hunting reports in our database.
We grouped the information obtained from the interviews into five broad themes: socio-demographic data, river usage and sighting information (year, month, season and time of day), habitat type (flowing water or deep isolated pool), information on nesting (nesting months, nest and/or egg reports, nest characteristics) and records of dead turtles (bycatch, hunting or other cause; Table 1). Within each theme, we coded open answers into categories in a post hoc manner to facilitate analyses.
Table 1 Description of five themes included in the semi-structured interviews administered during June–December 2019 in six panchayats along Chandragiri River in Kerala, India (Fig. 1), regarding Cantor's giant softshell turtle Pelochelys cantorii.

Data analysis
The same interpreter who conducted the interviews translated the interview responses into English. We used logistic regression to determine the effect of river use and respondent age on the probability of sighting P. cantorii in the study area. We used the glm function from the stats package in R 4.3.1 (R Core Team, 2023) to run the binomial logistic regression. We performed model selection using the stepAIC function from the MASS package (Venables & Ripley, Reference Venables and Ripley2002). We performed model diagnostics using the DHARMa package (Hartig, Reference Hartig2022), to ensure that the data met the assumptions of linearity, homogeneity of variance and normality.
We explored the associations between frequency of sightings and habitat, time of the sighting and seasonality using Fisher's exact tests, having classified these nominal variables within two categories (‘seen’ and ‘not seen’; McDonald, Reference McDonald2014). We coded responses regarding the time of year when sightings occurred into ‘dry season’ (January–May) and ‘monsoon season’ (June–December). When respondents were uncertain about the time of the year when they had seen the species, we excluded the response from the analysis. Similarly, we used two categories for time of day: ‘daytime’ (05.00–17.00) and ‘night-time’ (17:01–04:49). We considered sightings at different times from the same respondent as separate entries for the analysis. Use of Chandragiri River varied between different sections of the river and between communities, with most respondents using the river for one or more of the following activities: subsistence fishing, irrigation, bathing and washing (clothes and livestock). As with time of sightings, we accounted for different uses as separate data points for the binomial logistic regression.
Results
Demographics
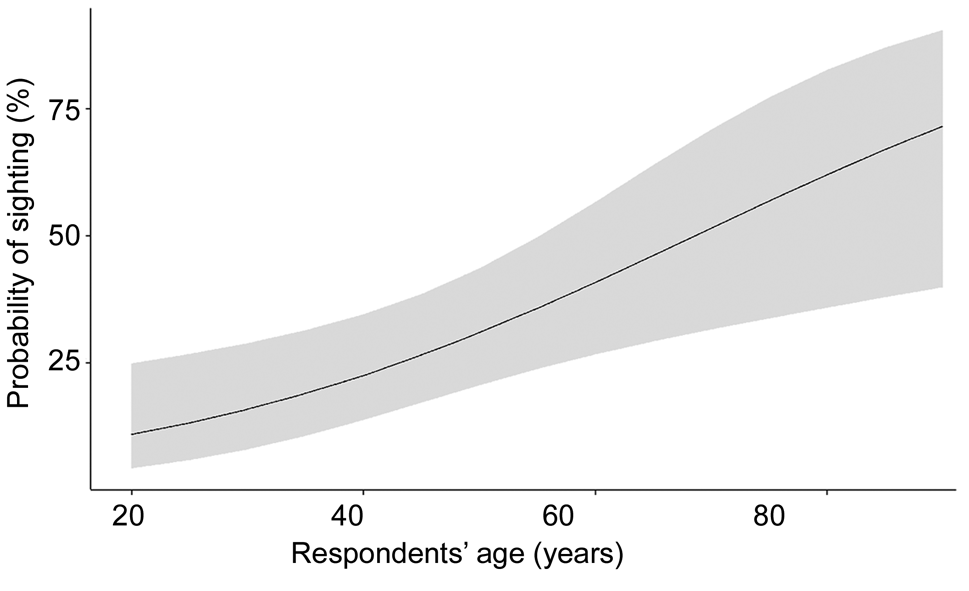
We completed 150 interviews, with 93 of these interviews instigated via opportunistic sampling. Respondent age ranged from 20 to 89 years (median = 49 years; Fig. 2). We interviewed more men (71%, n = 106) than women (29%, n = 44), as in most cases the male member of the family volunteered to respond during opportunistic sampling. Such cultural aspects leading to gender bias in our dataset could not be avoided with our sampling design. Most respondents were farmers (45%), followed by homemakers (persons involved in housekeeping; 24%) and daily wagers (persons involved in manual labour, including construction work, harvest and other activities; 23%). Eight per cent of respondents had more than one occupation or were retired.
Table 2 Results of logistic regression of the probability of sighting Cantor's giant softshell turtle with respondents’ age (Fig. 2) and the usage of the river (subsistence fishing and irrigation) in the surveyed panchayats of Kasaragod, Kerala, India.

Influence of river usage and respondent age on knowledge of the turtle
A total of 86 interviewees reported using Chandragiri River for one or more purposes, mainly for irrigation (n = 67), subsistence fishing (n = 26), washing (n = 20) and bathing (n = 8). Most respondents did not list fishing as their occupation; however, 30% of river users (n = 26) reported subsistence fishing. Model selection showed that age, subsistence fishing, irrigation and bathing were the top predictors of sighting P. cantorii (Supplementary Tables 1 & 2). However, we removed bathing from the final model because it was not statistically significant and respondents using the river for bathing also used it for other activities (Supplementary Table 3). Respondents using the river for subsistence fishing and irrigation had a greater probability of sighting the turtle (Table 2) than those who only used the river for washing. The probability of having seen P. cantorii also increased with respondent age (Fig. 2): 76% of respondents aged 60 years and above reported direct observation of P. cantorii, compared to only 28% of respondents below the age of 30 years.
Local ecological knowledge of P. cantorii
Only three respondents failed the validation question and could not discriminate P. cantorii from the other turtle species. Nearly half (48%; n = 72) of respondents reported having seen P. cantorii at least once in their lifetime. Of these respondents, 68% (n = 49) reported having seen the turtle only once, and 32% (n = 23) reported having seen it more than once. Most respondents (n = 61) reported sightings during 2010–2019, with almost half (n = 30) reporting sighting the species in 2019 (Table 3). From these, multiple respondents reported sightings in the same pool in 2019, suggesting that the same individual may have been observed in multiple sighting reports. Most respondents who had observed P. cantorii (81%, n = 58) reported having sighted the turtle in the afternoon during 12.00–15.00. The results from the Fisher's exact test also showed a statistically significant association between sighting of the species and time of the day (P < 0.001; Table 4), with the probability of sighting the species in the day being seven times higher than at night (odds ratio = 6.99, 95% CI = 3.73–13.62). We found no significant association between habitat type (isolated deep pools vs flowing rivers) and sighting of the species (P = 0.87, odds ratio = 0.89, 95% CI = 0.45–1.79).
Table 3 Reported sightings of Cantor's giant softshell turtle during 1970–2019 in the six surveyed panchayats of Kasaragod, Kerala, India.

Table 4 Fisher's exact test results regarding the association between sightings of Cantor's giant softshell turtle (seen and not seen) with habitat, time of the day and season in the six surveyed panchayats of Kasaragod, Kerala, India.

Seasonality
Sightings of P. cantorii were more frequent during the dry season (March–May), with 44% of all sightings (n = 32). There were eight reports of turtle sightings in the winter (November–February), only one respondent reported seeing the species during the south-west monsoon (in September), six reported sightings during the north-east monsoon (October–November), and more than one-third of respondents (n = 25) were unsure of when they had observed the turtle (Fig. 3). The results of the Fisher's exact test showed that the probability of sighting an individual was 11 times higher during the dry season than during the monsoons (P < 0.001, odds ratio = 11.22, 95% CI = 4.37–32.96; Table 4).

Fig. 3 The number of respondents (of a total of 72) who reported having sighted Cantor's giant softshell turtle across different seasons in the six surveyed panchayats in Kasaragod, Kerala, India. The category ‘Unsure’ comprises reports for which respondents were not certain of the season when the turtle was sighted.
Nesting
Ten per cent (n = 15) of respondents provided information on the nesting behaviour of the species, and all agreed that nesting occurs on the riverbanks. Six per cent of respondents (n = 9) could provide information on the nesting period (November–May). Three respondents provided detailed information about the nests. They reported that nests are 30–40 cm deep and eggs are laid in a spiral-chain fashion. One respondent recounted seeing c. 100 round, soft eggs within a nest.
Behaviour of P. cantorii
Ten informants reported that the species does not bask during the daytime; however, individuals raise their head above the water surface to breathe for a few seconds and then go back into deep waters (c. 5–10 m). Six informants believed the species to be highly secretive and shy and that it only comes out onto the riverbank at night. Six informants reported seeing the turtle jumping out of the water like a porpoise during the dry season. These informants were from the same community and used the same section of the river, and this behaviour was not reported in the other wards.
Hunting of P. cantorii
Approximately 15% of respondents (n = 23) reported catching the turtle at least once in their lifetime, and 13% (n = 19) reported targeted hunting of the species. Fewer than 3% (n = 4) of the respondents reported catching it as bycatch. In 87% (n = 20) of all catch incidents, the captured turtle was either consumed or sold locally for consumption. In all catch instances (bycatch or targeted), the turtles did not die whilst in the fishing gear but were killed deliberately. Only on two occasions were turtles confiscated by the State Forest Department after being found dead. Five informants told us that they used to hunt the species c. 2–3 decades ago when it was more abundant (a perception shared amongst these informants). These five informants ranged from 45 to 72 years old (median = 61 ± SD 10 years). Four of these informants continued to use the river for subsistence fishing and irrigation.
Alert network and its advantages
We established a network from the respondent pool by identifying individuals with substantial information on the study species who were willing to be key informants in their locality. We called this the ‘alert network’, and it comprised 35 key informants who lived near the study area. The motivation behind creating a local alert network was to involve local communities in data collection and to create a sustainable, locally led monitoring network for the species. So far, the network has resulted in 24 calls reporting nine direct and indirect sightings, seven bycatch events and six P. cantorii nests during 2019–2022. All of the bycaught turtles were rescued and released back to the catch locations. The reports obtained through the alert network confirmed the presence of a breeding population and provided information on the nesting ecology of the species (Plate 1).

Plate 1 (a) Adult Cantor's giant softshell turtle Pelochelys cantorii bycatch and (b) hatchling from a nest reported by the alert network in Kasaragod, Kerala, India.
Discussion
In this study, we gathered information on the elusive P. cantorii from local ecological knowledge of communities living adjacent to Chandragiri River in Kasaragod District, Kerala, India. Whereas past studies struggled to gather data on the species using ecological survey techniques (Sirsi, Reference Sirsi2010; Behera et al., Reference Behera, Panda, Dutta and Nayak2019), our study recorded multiple sighting reports (Jain et al., Reference Jain, Cavada-Blanco, Palot, Das, Deepak and Das2021) and critical ecological information, highlighting the importance of local ecological knowledge as a data source, and of community-based interviews to obtain such data. The data presented in our study will help to design future survey strategies for P. cantorii and to direct ongoing work to record ecological information that is critical for the species’ conservation. We found that only people who regularly use the river for subsistence fishing and irrigation were able to provide information on the species. This allowed us to establish an alert network and guide more effective collaboration with fishing and farming communities to facilitate long-term monitoring programmes for the species across this region. Our study establishes a framework for the use of this approach in other parts of the species’ range.
Our results confirm the presence of P. cantorii at the study site and suggest that it could be rare in Chandragiri River, with fewer than 50% of respondents being aware of the presence of P. cantorii. Furthermore, key informants perceived that the population had declined in recent decades, with fewer reports of bycatch in the years preceding the survey. However, the information gathered through interviews shows that most sightings occurred during 2010–2019 and predominantly in 2019. This could be the product of bias or a skewed number of interviewees within the age groups. Recollection bias can render local ecological knowledge inaccurate or incomplete, as interviewees usually cannot perfectly recall past events (Hamilton et al., Reference Hamilton, de Mitcheson, Aguilar-Perera, Sadovy de Mitcheson and Colin2012). Combined with a potential recency effect, by which interviewees mostly recall only the most recent past (Carothers et al., Reference Carothers, Brown, Moerlein, López, Andersen and Retherford2014), this could have increased the frequency of reported sightings during the time just prior to the survey. As sightings of P. cantorii are relatively rare in the study area, respondents may only recall their most recent sighting(s). This bias was potentially increased by the news coverage of a mass die-off of fish within an isolated deep pool of Chandragiri River in May 2019 (Nair et al., Reference Nair, Tholkudiyil and Shaji2021). Although the lack of knowledge regarding the biology and ecology of P. cantorii limits our capacity to validate the information obtained from local people, we believe it is reliable. We gathered multiple corroborating accounts of bycatch, sightings and nesting, giving us confidence in the validity of the data.
Another form of bias could have arisen from the local naming of the species. Pelochelys cantorii is known across the study area as paala poovan. This name derives from a local name for the flower of the species Tabernaemontana heyneana in Malayalam. According to local communities, the snout of this turtle species resembles the stigma of T. heyneana, and the white colour of the flower is equated to the white plastron of the turtle. A similar name of paale poo is used in the neighbouring state of Karnataka for Leith's softshell turtle Nilssonia leithii (Sirsi, Reference Sirsi2010). In the study area the name paala poovan is reportedly only used for P. cantorii; however, reporting bias could occur if respondents confuse the two species. We addressed this bias by asking the respondents to identify the species from the photographs of different turtle species, including N. leithii.
Most informants reported that individuals of P. cantorii are frequently seen in deep pool areas, especially during the dry season. However, the Fisher's exact test showed that sightings were independent of habitat type (isolated deep pool vs flowing river). It has been previously observed that the species inhabits deep pool areas in the Mekong River in Cambodia (Emmett, Reference Emmett2009), and similar accounts were shared by the local communities in Odisha, India (Behera et al., Reference Behera, Panda, Dutta and Nayak2019). It is unclear whether the species prefers one habitat type over the other or whether these observations can be attributed to habitat availability. Our results could be biased because of the small sample size used in the test. It is also possible that some respondents misinterpreted the question about habitat type (providing habitat information only relating to their most recent turtle sighting), and hence the data could be biased. In future surveys, focus groups with key informants could be used to address this issue.
Our analysis suggests that there is a strong positive association between the dry season and sightings of P. cantorii. This could be influenced by the use patterns of the river and adjacent areas by respondents as well as seasonal changes that could alter sighting probability. Information from the interviews suggests that sightings of the turtle become more likely as the water begins to recede in mid December and increase further as the river level falls during the dry season. Contrasting with reports of sightings in estuarine habitats during the monsoons (Palot & Radhakrishnan, Reference Palot and Radhakrishnan2011), respondents reported that it was rare to sight this species in the river or estuarine areas during the south-west monsoon. None of our key informants could provide information on the movement of P. cantorii during the monsoons, but some hypothesized that the species might move towards the sea or take refuge in deeper sections of the river.
The surveys show that the species is seen mainly during afternoon hours. The turtles were always observed in the water and never basking on the riverbanks. Consequently, informants reported that the species only comes out onto the riverbanks at night. Reports of sightings during night-time were few, possibly because the study area is sometimes visited by elephants and therefore community members do not frequently visit the river at night. This could explain why most sightings were made during the afternoon in the study area.
The nesting and breeding ecology of P. cantorii is poorly studied. It has been previously suggested that in India the species moves towards estuarine habitats during August–October to breed (Palot & Radhakrishnan, Reference Palot and Radhakrishnan2011), but nesting has also been reported to occur during December–March (Behera et al., Reference Behera, Panda, Dutta and Nayak2019). Only a few of the informants in our study had any knowledge of the nesting ecology of P. cantorii. We also received contrasting information on the nesting period, with one informant reporting nesting during November–December, five reports of nesting during January–March and three reports of nesting during April–May. However, the key informants reported nesting of the species to have occurred consistently during January–February over 3 years (2020–2022). Our team has monitored these nests, confirming the nesting period of the species to be during January–February in Kerala (Jain et al., unpubl. data).
Although local ecological knowledge provides essential insights into species ecology, there are limitations to this approach. Clarifying the purpose of each question in the interview is of paramount importance. If respondents misinterpret a particular question, it could change the information provided or introduce bias (Brook & McLachlan, Reference Brook and McLachlan2005; Hamilton et al., Reference Hamilton, de Mitcheson, Aguilar-Perera, Sadovy de Mitcheson and Colin2012). For example, when asked about the habitat preference of P. cantorii, most respondents only provided habitat information for their most recent sighting of the species. To address this, we collected information in an open-ended format, wherein respondents were given additional context for each question they did not fully understand. The interviewer's level of familiarity with the respondents can also influence the responses (Brook & McLachlan, Reference Brook and McLachlan2005), which was apparent in some cases during our study. We observed that respondents appeared more comfortable sharing information when we were accompanied by a local person who was familiar to them. Additionally, our sampling methods (snowball and opportunistic sampling) could have introduced selection biases. As the study area is culturally diverse and religiously demarcated, snowball sampling may have led to over- or under-representation of different groups depending on the initial interviewee who provided referrals. During opportunistic sampling we only targeted panchayats and areas close to Chandragiri River and assumed that communities distant from the river would be unaware of the species. This could have influenced our results and a more representative survey is required to address this potential bias.
In the study area, P. cantorii is threatened by the consumption of its meat, despite its protected status and the penalties for hunting, trading and consuming the species. Although the species does not have any particular nutritional or medicinal benefit (Emmett, Reference Emmett2009) and the quality of its meat is poor (Shepherd, Reference Shepherd2000), in some places the meat of P. cantorii is believed to cure paralysis and dyspnoea (Rahman et al., Reference Rahman, Mamun, Rahman, Hossain, Minar and Maheen2013). Our findings suggest that in the study area, the species is consumed only for its meat and only opportunistically, with such consumption having no associated cultural or medicinal values. It was notable from the interviews that the turtles, whenever caught, were either consumed or sold locally. However, these reports may not reflect the true levels of hunting and consumption, given that many respondents may have perceived the questions related to these activities as sensitive. Many studies have reported the consumption of P. cantorii meat (Das, Reference Das2008), with a lack of awareness amongst local people (both communities and governing authorities) and inadequate enforcement of laws outside protected areas resulting in continued hunting of threatened turtles in India (Bhupathy et al., Reference Bhupathy, Choundry, Hanfee, Kaylar, Khan, Platt and Rashid2000). The calls for turtle rescues made during our study through the alert network suggest that awareness amongst local communities regarding the species and its legal protection can reduce killing through bycatch or targeted hunting. In addition, proper training of forest officers and strict enforcement of existing laws could potentially reduce this threat (Choudhury, Reference Choudhury2001).
The importance of local knowledge for biodiversity monitoring has been increasingly recognized over the past 2 decades. Local ecological knowledge has provided valuable information regarding rare and/or possibly extinct species in cases where expensive ecological survey methods have been ineffective (Johannes et al., Reference Johannes, Freeman and Hamilton2000; Turvey et al., Reference Turvey, Risley, Moore, Barrett, Yujiang and Xiujiang2013). For instance, local ecological knowledge has been key to determining the status of the Critically Endangered Chinese giant salamander Andrias davidianus (Pan et al., Reference Pan, Wei, Cunningham, Li, Chen, Milner-Gulland and Turvey2016; Turvey et al., Reference Turvey, Chen, Tapley, Liang, Wei and Yang2021). Similarly, the information gathered from key informants in this study has provided critical information on P. cantorii nests and active nesting sites, considerably reducing the sampling effort in our ecological surveys. In combination with field surveys, local knowledge can also help with species assessments (Kanagavel & Raghavan, Reference Kanagavel and Raghavan2012), and to identify key informants who can play a critical role in the long-term conservation of the species in the study area.
The alert network established through our study initiated the first ever community-based plan directed at conserving freshwater turtles in India, through which people voluntarily report sightings, nesting information and bycatch of the species. These reports have enabled us to identify priority conservation areas and subsequently to identify threats to these priority areas, including illegal sand mining, construction of dams causing flooding of nesting grounds, and fishing and agricultural pressures, reinforcing the need for immediate conservation intervention (Jain et al., unpubl. data).
The results of this study have enabled us, in collaboration with local governments, to conduct multiple training and awareness sessions (January 2020, January 2021, March 2021) with the key informants on the rescue and safe release of bycaught turtles. We have also established helpline numbers for anonymously reporting hunting activity and inundated nests, and have initiated efforts to hire local community members to participate in nest protection programmes. These initiatives are ongoing in the study area, and we recommend that they are continued to ensure the long-term monitoring and conservation of the P. cantorii population. Establishing trust and shared knowledge between local communities and governments can be effective for managing threatened species outside protected area networks. Our study therefore demonstrates the importance and value of local ecological knowledge for understanding and addressing conservation issues for globally threatened and elusive species. Wide-scale incorporation of this approach to gathering ecological data on P. cantorii could fill knowledge gaps and help to reduce the extinction risk that this species faces.
Author contributions
Project conceptualization: AJ, VD, AD, FC-B; study design: AJ, FC-B; data collection, fieldwork: AJ, VAA; data analysis: AJ, VAA, PB, FC-B; writing: AJ, FC-B, with contributions from all authors.
Acknowledgements
We thank the EDGE of Existence Programme at the Zoological Society of London and the National Geographic Society for funding this research through an EDGE PhotoArk Fellowship awarded to AJ; the Mohamed Bin Zayed Species Conservation Fund for support with nesting surveys and protection; the Kerala Forest Department and Kerala State Biodiversity Board for their support and permission to carry out the study (KFDHQ-28470/2018-CWW/WL10); the Wildlife Institute of India for providing in-country institutional support; the local community members and governing bodies for their contributions and for sharing their knowledge; Cassandra Murray for providing valuable insights during the conceptualization of the study; the Zoological Survey of India, Jafer Palot and Sandeep Das for their continued support in Kerala; Balakrishnan Nair for logistical support; Mithun M.V., Vishnu Nair and Julsana Jalal for field assistance and translation; The Habitats Trust for providing funds to continue the project in the study area; and the reviewers and Editor for their comments on the text.
Conflicts of interest
None.
Ethical standards
All methods used were reviewed and approved by the EDGE of Existence Programme and followed its ethical guidelines, which have been developed following the guidelines of the British Sociological Association and the Social Research Association. The research also abided by the Oryx guidelines on ethical standards.
Data availability
Anonymized data and the scripts used for the analyses are available at github.com/AyushiJain96/Logisticregression.