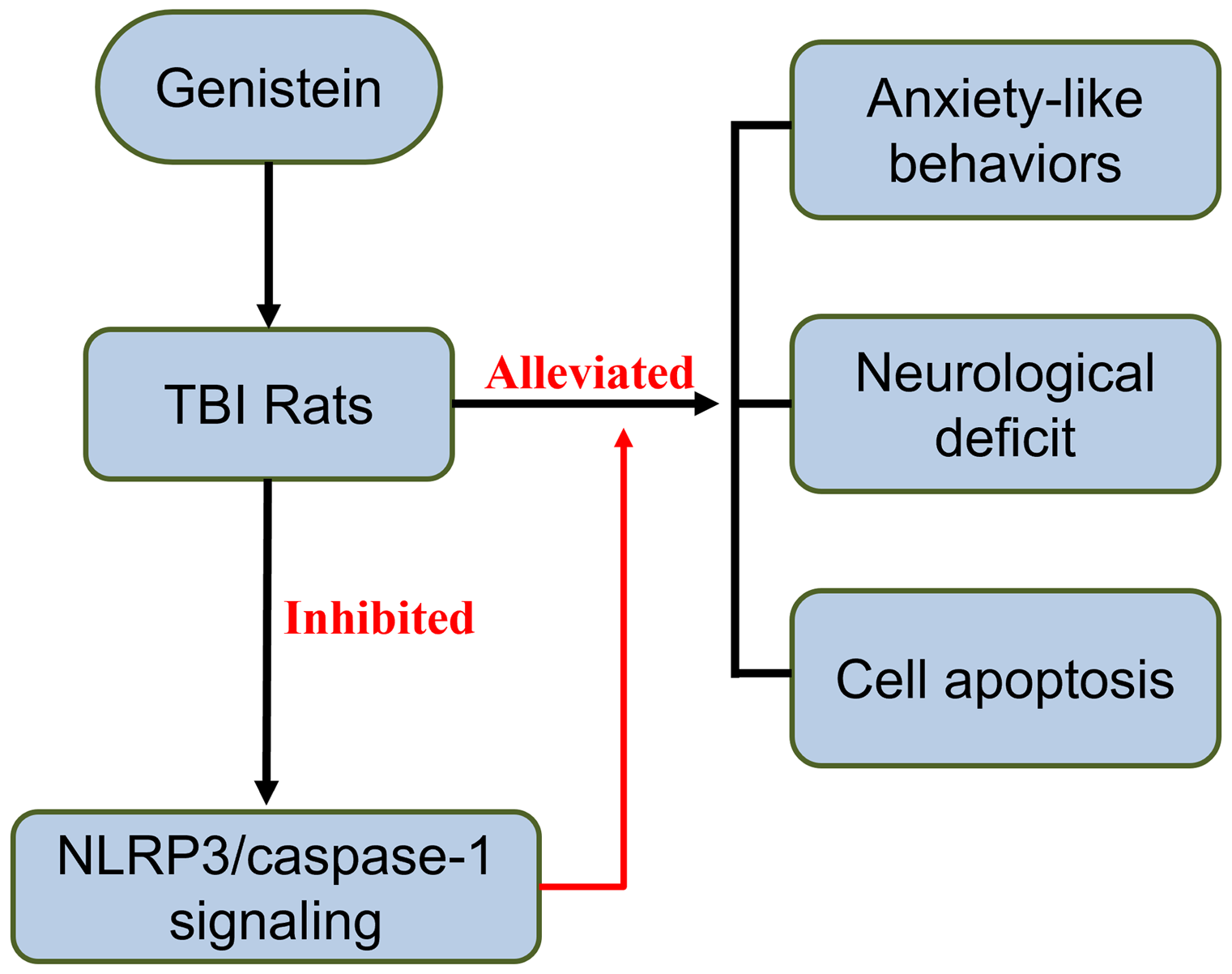
Significant outcomes
-
Genistein alleviates anxiety caused by traumatic brain injury.
-
Genistein improves neurological deficits and related impairments.
-
Genistein is beneficial for neural impairments.
Limitations
-
Only the TBI animal model was employed in the current study; other animal models of brain injury rather than TBI could be used.
-
The detailed molecular mechanisms underlying the protective effects of genistein could be further explored using omics, such as RNA sequencing.
Introduction
Traumatic brain injury (TBI), which is one of the most common causes of death worldwide, is characterised by non-degenerative, acquired structural damage and/or brain dysfunction caused by an external force, leading to alterations in the level of consciousness, and long-term or transient disability of cognitive and physical functions (Shahim and Zetterberg, Reference Shahim and Zetterberg2021). Neuropsychiatric disorders, including anxiety, depression, and psychosis, are common consequences of TBI. Owing to advances in treatment strategies, there is a significant decrease in the mortality rate of TBI, but a tremendous gap exists in the clinical management of neuropsychiatric detriments caused by TBI (Rabinowitz and Watanabe, Reference Rabinowitz and Watanabe2020). There is an urgent need to develop novel approaches to alleviate TBI-related psychiatric disorders (Traeger et al., Reference Traeger, Hoffman, Misencik, Hoffer and Makii2020).
Inflammation is a central driver of TBI-induced psychiatric impairments (Risbrough, et al., Reference Risbrough, Vaughn and Friend2021). Following the direct mechanical brain damage, irreversible neuronal death occurs, which comprises the primary damage caused by TBI. Subsequently, a cascade of molecular, biochemical, and cellular changes, including predominantly inflammatory responses and free radical generation, was initiated that further exerts deleterious effects on neurone physiology for hours after TBI, which comprises secondary damage by TBI (Vedantam et al., Reference Vedantam, Brennan, Levin, McCarthy, Dash, Redell, Yamal and Robertson2021). Hence, recent studies have largely explored anti-inflammatory drugs to reduce TBI-induced brain inflammation (Kalra et al., Kalra et al., Reference Kalra, Malik, Singh, Bhatia, Al-Harrasi, Mohan, Albratty, Albarrati and Tambuwala2022). One of the anti-inflammatory drugs commonly used for neurological disorders is genistein, a plant-derived isoflavone found in soy foods (Duan et al., Reference Duan, Li, Xu and Ding2021). The potent effects of genistein in reducing neurological damage have been demonstrated in its role in alleviating ischaemic injury in the brain (Oppong-Gyebi et al., Reference Oppong‐Gyebi, Metzger, Doan, Han, Vann, Yockey, Sumien and Schreihofer2022), stroke (Schreihofer and Oppong-Gyebi, Reference Schreihofer and Oppong-Gyebi2019), Alzheimer’s disease (Duan et al., Reference Duan, Li, Xu and Ding2021), etc. For example, it was shown that genistein delayed the onset of mortality and disability in a model of amyotrophic lateral sclerosis (Zhao et al., Reference Zhao, Fu, Li and Li2019). A previous study also provided preliminary testing showing that genistein can be neuroprotective in TBI (Soltani et al., Reference Soltani, Khaksari, Jafari, Iranpour and Shahrokhi2015). Similarly, genistein was reported to attenuate brain oedema, blood-brain barrier disruption, and aberrant neurobehavioural performances (Soltani et al., Reference Soltani, Khaksari, Jafari, Iranpour and Shahrokhi2015). However, whether genistein treatment could ameliorate TBI-induced neuropsychiatric disorders remains unknown.
Nod-like receptor protein 3 (Nlrp3), a recently discovered inflammasome, was found to play a key role in mediating inflammatory responses in a variety of neurological disorders including ischaemic stroke (Alishahi et al., Reference Alishahi, Farzaneh, Ghaedrahmati, Nejabatdoust, Sarkaki and Khoshnam2019). More importantly, a previous study suggested that t genistein could attenuate acute cerebral ischaemic damage by inhibiting Nlrp3 inflammasome (Wang et al., Reference Wang, Wang, Wei, Gu, Wang, Wu and Yang2020). The current study focused on the regulation of Nlrp3 signalling by genistein and explored its protective effects against TBI-induced psychiatric disorders.
Materials and methods
TBI model
In this study, adult male Sprague-Dawley rats with a weight of 300–350 g were used. TBI models were constructed using the lateral fluid percussion injury (LFPI) method for inducing both focal and diffusive injuries to the brain. The LFPI method is widely used in research to induce TBI in animal models because it recapitulates many of the pathophysiological changes observed in human TBI. It’s a well-established method that produces injury by a brief fluid pressure pulse applied directly to the exposed dura mater over the intact skull, simulating the mechanics of human brain injury (Alder et al., Reference Alder, Fujioka, Lifshitz, Crockett and Thakker-Varia2011). A total of 96 rats were used and housed in groups of 2 per cage. Briefly, the animals were fixed on a stereotaxic table, routinely disinfected and towelled, and the skin and periosteum were cut in layers in the middle of the head to expose the right parietal bone, and the rat’s brain was positioned at 3 mm next to the sagittal suture and 3.5 mm behind the coronal suture. A hole was drilled in the skull with a craniotomy drill to expose the meninges, and a small cap of the same size as the hole was placed on the dura mater. After fixing the hydraulic tube, 3 atm (standard for heavy craniocerebral injury) was administered, and the sham operation group only opened the bone window without impact. For the behavioural tests and brain water examination, a total of 60 rats with 12 rats per group were used. For the following experiments, a total of 36 rats with 12 rats per group were used. Animal studies were approved by the Ethics Committee of Xingtai Medical College.
Genistein treatment
Genistein was dissolved in dimethyl sulfoxide to a concentration of 200 mg/ml and then diluted to 1 mg/ml with a buffer containing 50% saline, 10% tween-80, and 40% PEG300. Rats were intraperitoneally injected with genistein at the dose of 5, 10, and 20 mg/kg, following the published literature (Soltani et al., Reference Soltani, Khaksari, Jafari, Iranpour and Shahrokhi2015; Wang et al., Reference Wang, Wang, Wei, Gu, Wang, Wu and Yang2020; Wang et al., Reference Wang, Zhang, Wang, Ma, Zhao, Wang, Fang, Hou and Guo2022), or the same volume of vehicle (buffer only).
Genistein treatments were performed in two rounds. In the first round, the rats were treated with genistein once daily for 14 consecutive days after TBI. Following TBI induction, the animals were subjected to behavioural tests. Before the operation, the evaluation of neurological deficit was performed at 1, 3, 7, and 14 days after TBI. At 12 days after TBI, an elevated plus maze (EPM) experiment was performed. At 13 days after TBI, an open field test (OFT) was performed. In the second round, genistein treatment was started at 30 min, 12 h, 24 h, 48 h, and 72 h after TBI. At the end of the treatment, the rats were euthanised, and their brains were harvested for biochemical assessment.
Neurological deficit evaluation
Neurological deficit evaluation was conducted using a modified neurological severity score (mNSS) as described in a previously reported protocol (Yuan et al., Reference Yuan, Wang, Liu, Chen, Zhang, Shen, Liu and Fu2018). The severity scores were determined as follows: 0: no dysfunctions; 1–6 points: mild damage; 7–12 points: moderate damage; and 13–18 points: severe damage. The higher the mNSS score is, the more severe the damage to the brain injury.
OFT and EPM test
At 12 days after TBI, an EPM experiment was performed. Locomotion activity was quantified using OFT (Aravind et al., Reference Aravind, Ravula, Chandra and Pfister2020) in an activity chamber, where rats were positioned in the centre of the field, followed by recoding locomotive activity for 5 min. Assessments included measuring the total time in and outside the centre zone, the time duration spent by the walls, and the frequency of the centre cross. Anxiety manifested as reduced frequency of the centre cross and decreased distance travelled and velocity.
An EPM (Lengel et al., Reference Lengel, Huh, Barson and Raghupathi2020) was also used to evaluate anxiety and explorative behaviours according to established protocols. Briefly, the movement of animals was recorded after they were placed in a maze consisting of open and closed arms. Rime ratio (RT), the metric that evaluates anxiety, represents the ratio of time spent in the open arms (TO)/sum of time spent in both closed (TC) and open arms (TO): RT = TO/(TO + TC).
Measurement of brain water content
As a parameter reflecting brain oedema, the water content of brain tissues was measured by the wet/dry method (Thomas et al., Reference Thomas, Oros‐Peusquens, Schöneck, Willuweit, Abbas, Zimmermann, Felder, Celik and Shah2023). Briefly, brain tissues were quickly removed after euthanisation, and brain tissues were sectioned into 4 mm thick slices. After acquiring the wet weight (WW), the slices were weighed after 24 h at 100°C to yield the dry weight (DW). The water content of brain tissue (%) was calculated as [WW-DW]/WW × 100%.
TUNEL staining
TUNEL staining was used to evaluate apoptosis in the brain. Cells fixed in paraformaldehyde were permeabilised with sodium citrate and Triton X-100 and then incubated with the TUNEL reaction mixture according to the manufacturer’s protocol (Roche Diagnostics). Post-incubation, the samples were rinsed and counterstained with DAPI to visualise nuclei. The proportion of TUNEL-positive cells relative to the total number of cells was then quantified using fluorescence microscopy.
ELISA
After homogenising the cortical tissues and centrifugation at 10,000 g for 15 min, the supernatant was collected, and ELISA kits (Nanjing Jiancheng Bioengineering Institute) were used to measure levels of the inflammatory cytokines according to the manufacturer’s protocols.
Real-time PCR
Real-time PCR was performed after extracting total RNAs from brain tissue using the TRIzol kit (Thermo Fisher Scientific), and the RNAs were reversely transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit from Applied Biosystems. The following primers were used: Asc: F: GATGCCATCCTGGACGCTCTTG, R: ATGAGTGCTTGCCTGTGTTGGTC; Nlrp3: F: CTGCGGACTGACCCATCAATGC, R: ACCAATGCGAGATCCTGACAACAC; GAPDH: F: GATGCCCCCATGTTTGTGAT, R: GGCATGGACTGTGGTCATGAG; Caspase-1: F: GCCGTGGAGAGAAACAAGGAGTG, R: GGTCACCCTTTCAGTGGTTGGC. Gene expression quantification was achieved using the ΔΔCt method, normalising against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the housekeeping gene.
Western blot
The protein content of brain homogenates was measured using the BCA assay kit (Beyotime). Then, the proteins were resolved by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The 5% non-fat milk was used for membrane blocking for 1 h at room temperature. The primary antibodies against Nlrp3, Asc, pro-caspase-1, caspase-1 (p20), and GAPDH (all acquired from cell signalling) were then applied to the membranes and shaken at 4°C overnight. Fluorescent dye-labeled secondary anti-rabbit or mouse antibodies incubated with the membrane for 2 h at room temperature. Protein bands were visualised and quantified using a fluorescence imager.
Statistical analysis
All data were expressed as the mean with standard deviation (mean ± SD). One-way analysis of variance (ANOVA) followed Dunn’s multiple comparisons test were used. P < 0.05 was considered statistically different. The significance levels were denoted as ***p < 0.001 compared to sham. ^p < 0.05, ^^p < 0.01, and ^^^p < 0.001 compared to vehicle.
Results
Genistein alleviates TBI-induced anxiety-like behaviours in rats
Immediately after TBI induction, the rats were treated with genistein once daily for 14 consecutive days. As shown in Figure 1a–d, OFT results suggested that there were significant decreases in the numbers of the centre cross (Fig. 1a, Sham: 34.83 ± 4.32, Vehicle: 18.33 ± 2.27, Gen5: 22.17 ± 3.19, Gen10: 24.47 ± 4.31, Gen20: 28.42 ± 4.14), distance travelled (Fig. 1b, Sham: 31.16 ± 3.81 m, Vehicle: 19.57 ± 3.63 m, Gen5: 23.83 ± 3.45 m, Gen10: 25.18 ± 3.91 m, Gen20: 27.25 ± 4.02 m), velocity (Fig. 1c, Sham: 15.65 ± 1.64 cm/s, Vehicle: 8.11 ± 1.57 cm/s, Gen5: 10.06 ± 1.77 cm/s, Gen10: 11.22 ± 2.07 cm/s, Gen20: 13.32 ± 1.94 cm/s) and a significant increase in immobility (Fig. 1d, Sham: 43.26 ± 7.69 s, Vehicle: 98.86 ± 12.14 s, Gen5: 84.57 ± 8.16 s, Gen10: 75.65 ± 10.33 s, Gen20: 60.12 ± 8.78 s) comparing vehicle group. Moreover, the effects of genistein treatment demonstrated a dose-dependent pattern, and it had the best effects at the doses of 20 mg/kg.

Figure 1. Genistein alleviated traumatic brain injury-induced anxiety-like behaviours in open field tests. Genistein increased the number of centre crosses ( a ), the total distance travelled ( b ), and also the velocity ( c ). Genistein decreased the immobility time ( d ). n = 12 for each group.
Similarly in the EPM test, TBI rats exhibited anxiety-like behaviours including increased time in the centre (Fig. 2a, Sham: 26.82 ± 5.12 %, Vehicle: 42.25 ± 6.89 %, Gen5: 38.84 ± 6.11 %, Gen10: 34.56 ± 5.88 %, Gen20: 31.45 ± 5.67 %), decreased time in distal open arms (Fig. 2b, Sham: 12.76 ± 2.13 %, Vehicle: 6.23 ± 1.22 %, Gen5: 7.82 ± 1.21 %, Gen10: 8.72 ± 1.74 %, Gen20: 11.08 ± 1.67 %), decreased open/close arms ratio (Fig. 2c, Sham: 0.41 ± 0.07, Vehicle: 0.22 ± 0.05, Gen5: 0.28 ± 0.03, Gen10: 0.32 ± 0.06, Gen20: 0.37 ± 0.07), and reduced total travelled distance (Fig. 2d, Sham: 24.37 ± 4.56 m, Vehicle: 13.24 ± 2.31 m, Gen5: 16.13 ± 2.14 m, Gen10: 17.31 ± 2.57 m, Gen20: 19.46 ± 2.62 m). Genistein treatment, however, led to a reduction in the time in the centre and increases in the time in the open arms, open/closed arms ratio, and total travelled distance in a dose-dependent manner, while genistein at the dose of 20 mg/kg exhibited the most robust effects.

Figure 2. Genistein alleviated traumatic brain injury-induced anxiety-like behaviours in an elevated plus maze test. ( a ) Genistein decreased the time spent in the centre. ( b ) Genistein increased the time spent in the distal parts of the open arms and the time spent on the open arms ( c ) as well as the total distance travelled ( d ). n = 12 for each group.
Effects of genistein on TBI-induced neurological deficit and brain oedema
Neurological deficit evaluation was performed preoperatively, on days 1, 3, 7, and 14 after TBI. Our data showed that while mNSS increased in all rats with TBI, a faster decline was seen in rats treated with genistein, and higher doses of genistein induced a significantly faster decline (Fig. 3a). Further, measurement of brain water content suggested that the substantial increase in brain oedema after TBI was pronouncedly attenuated by genistein treatments dose dependently (Fig. 3b, Sham: 78.12 ± 1.30%, Vehicle: 84.73 ± 1.79%, Gen5: 82.14 ± 1.39%, Gen10: 81.46 ± 1.61%, Gen20: 80.09 ± 1.59%). Together, these data implicated that genistein has a significant ameliorative effect on TBI and its subsequent anxiety-like behaviours. To avoid excessive sacrifice of animals, we used only the most effective concentration of 20 mg/kg in the following experiments.

Figure 3. Effects of genistein on traumatic brain injury-induced neurological deficit ( a ) and brain oedema ( b ) in rats. Brain water content in the lesioned cortex was compared 3 days post-TBI (n = 8 for each group), and neurological deficit scores were measured pre-TBI and at 1, 3, 7, and 14 days post-TBI (n = 12 for each group).
Genistein alleviates TBI-induced cell apoptosis
We further collected brain tissues from rats 14 days after TBI and conducted TUNEL staining to evaluate cell apoptosis in brain cortices. The data suggested that TBI led to a marked increase in apoptotic cells and a dramatic reduction of apoptotic cells could be observed in the brain cortices after being treated with genistein at 20 mg/kg (Fig. 4, Sham: 2.26 ± 0.32%, Vehicle: 32.43 ± 3.57%, Gen20: 17.35 ± 2.11%).

Figure 4. Genistein alleviated traumatic brain injury-induced cell apoptosis in the lesioned cortices tissues of experimental rats. The ratios of TUNEL-positive cells in different groups. n = 6.
Genistein alleviates TBI-induced inflammatory responses
Given the important role of inflammation in inducing anxiety-like behaviours, the production of inflammatory cytokines, including IL-1β, TNF-α, and IL-18 in the brains of experimental rats, was determined using ELISA. The levels of IL-1β (Fig. 5a, Sham: 21.13 ± 3.65 pg/mg tissue, Vehicle: 161.77 ± 12.91 pg/mg tissue, Gen20: 78.98 ± 17.83 pg/mg tissue), TNF-α (Fig. 5b, Sham: 15.65 ± 3.11 pg/mg tissue, Vehicle: 128.38 ± 18.66 pg/mg tissue, Gen20: 61.22 ± 15.24 pg/mg tissue) and IL-18 (Fig. 5c, Sham: 35.21 ± 5.45 pg/mg tissue, Vehicle: 215.74 ± 27.99 pg/mg tissue, Gen20: 119.88 ± 18.25 pg/mg tissue) were significantly increased in the TBI rats, while their levels were significantly down-regulated after treatment with genistein at 20 mg/kg, suggesting that genistein was effective in suppressing inflammatory responses in the brain after TBI.

Figure 5. Genistein alleviated traumatic brain injury-induced inflammatory responses in the lesioned cortices tissues of experimental rats. Protein levels of IL-1β ( a ), TNF-α ( b ), and IL-18 ( c ) in the lesioned cortices tissues were measured by ELISA. n = 6 mice for each group.
Genistein inhibits Nlrp3/caspase-1 signalling pathway
To further explore the mechanism of genistein in treating TBI-induced anxiety, we analysed the expression levels of key members of the NLRP/caspase-1 signalling pathway, including Nlrp3, Asc, caspase-1, and pro-caspase-1, using qRT-PCR and Western blot. The messenger RNA (mRNA) expression of Nlrp3, Asc, and caspase-1 was significantly up-regulated in the TBI group (Fig. 6a–c), while genistein treatment at the dose of 20 mg/kg could down-regulated the expression of these genes. Consistently, the protein expressions of Nlrp3, Asc, caspase-1, and pro-caspase-1 were also significantly increased in the TBI group, and 20 mg/kg genistein effectively decreased their expression (Fig. 6d–h). These results demonstrate that genistein could potently suppress the activation of NLRP/caspase-1 signalling.

Figure 6. Genistein inhibited Nlrp3/caspase-1 signalling in the lesioned cortices tissues of experimental rats after traumatic brain injury. RT-qPCR was used to measure the mRNA expressions of Nlrp3 ( a ), Asc ( b ), and Caspase-1 ( c ) in the lesioned cortices tissues of experimental rats. Western blotting was used to measure the protein expressions of Nlrp3, Asc, pro-caspase-1, and caspase-1 in the lesioned cortices tissues from different groups ( d ). GAPDH was used as a loading control, and the expressions were normalised to Sham ( e – h ). n = 3 repeats were performed for each group (six tissue homogenates were mixed for each group). The number 3 represents three repetitions of mixed tissue homogenates, sourced from six rats’ tissues.
Discussions
Despite tremendous efforts devoted to alleviating the primary damage exerted by TBI, the detrimental effects on the neuropsychiatric wellness of the patients ensuing TBI are often overlooked (Brett et al., Reference Brett, Kramer, Whyte, McCrea, Stein, Giacino, Sherer, Markowitz, Manley, Nelson, Badjatia, Boase, Barber, Bodien, Bullock, Chesnut, Corrigan, Crawford, Diaz-Arrastia and Zafonte2021). To address the gap in managing the neuropsychiatric detriments caused by TBI, our study showed that genistein treatment could serve as a potential pharmacologic option to attenuate anxiety-like behaviours, decrease apoptosis in the neuronal tissues, improve the neurological scores, and reduce the release of inflammatory factors. Neuroinflammation is increasingly recognised as a critical factor in the pathophysiology of anxiety disorders (Won and Kim, Reference Won and Kim2020). Studies indicate that neuroinflammatory processes can lead to alterations in brain regions that are central to mood regulation and anxiety, such as the amygdala and prefrontal cortex (Guo et al., Reference Guo, Zhang, Hao, Wang, Zhang and Liu2023). Our findings are consistent with previous studies showing that genistein, as an anti-inflammatory drug, has neuroprotective efficacies in a plethora of neurological injuries and disorders (Miao et al., Reference Miao, Xia, Che and Song2018; Fuloria et al., Reference Fuloria, Yusri, Sekar, Gan, Rani, Lum, Ravi, Subramaniyan, Azad, Jeyabalan, Wu, Meenakshi, Sathasivam and Fuloria2022), and could be used as a potent drug to treat TBI. It is worth noting that the LFPI model, used in our study, induces mixed focal and diffuse injury, which recapitulates human TBI cases that are mostly mixed injury, granting greater translatability for our findings (Carron et al., Reference Carron, Sun, Shultz and Rajan2020). Our study represents one of the first studies that utilised behavioural, biochemical, and cellular evaluations to support the pharmacological potential of genistein in the neuropsychiatric aspect of TBI. Compared to most pharmacological interventions that are specifically intended to address psychiatric problems after TBI, which interact with neurotransmitter systems, such as dopamine and other anti-depressive drugs (Rabinowitz and Watanabe, Reference Rabinowitz and Watanabe2020), the combined efficacies of genistein in attenuating both primary injuries by TBI and the following psychiatric abnormalities make genistein a potentially powerful treatment in the clinical setting.
In addition, genistein also inhibited the Nlrp3 inflammasome, a central mediator of cellular damage injury and inflammatory responses, as evidenced by the decrease in mRNA and protein expression of Nlrp3, Asc, pro-caspase-1, and cleaved caspase-1 in the brain. Previously, Nlrp3 inflammasome has been identified as an important diagnostic biomarker and treatment target in TBI (O’Brien et al., Reference O’Brien, Pham, Symons, Monif, Shultz and McDonald2020). During inflammatory responses, the activation of Nlrp3 protein led to its interaction with Asc and pro-caspase-1, forming the Nlrp3 inflammasome. Further, the transformation of the pro-caspase-1 to caspase-1 induced by Nlrp3 inflammasome catalyses the production of inflammatory cytokines. Our results indicate that the anti-inflammatory role of genistein could play a critical role in its neuroprotective efficacies in TBI, and the efficacies of genistein stem at least partly from Nlrp3 inflammasome inhibition. Here we did not specify the type of cells responsible for Nlrp3 overexpression. As suggested by previous studies, Nlrp3 inhibition by genistein in microglia cells is primarily responsible for genistein’s neuroprotective effects in ischaemic stroke.
Given the promise of genistein in treating TBI-induced anxiety shown in our study, further exploration of genistein in other animal models of injury-induced neuropsychiatric impairments is warranted. In addition, testing the efficacy of genistein using other routes of genistein administration, for example, oral gavage and intravenous injection, would facilitate its clinical translation. Since genistein is already being tested in clinical settings (Marini et al., Reference Marini, Minutoli, Polito, Bitto, Altavilla, Atteritano, Gaudio, Mazzaferro, Frisina, Frisina, Lubrano, Bonaiuto, D'Anna, Cannata, Corrado, Adamo, Wilson and Squadrito2007; Lazarevic et al., Reference Lazarevic, Boezelijn, Diep, Kvernrod, Ogren, Ramberg, Moen, Wessel, Berg, Egge-Jacobsen, Hammarstrom, Svindland, Kucuk, Saatcioglu, Taskèn and Karlsen2011) with proven biocompatibility, we envision that genistein portends a shorter clinical path and can potentially be used as a post-traumatic drug for managing TBI patients.
Conclusions
In conclusion, the current study demonstrates the potency of genistein in attenuating anxiety-like behaviours, as well as ameliorating brain injury pathologically. It is also shown that genistein suppresses inflammatory responses in the injured brain and inhibits the Nlrp3/caspase-1 signalling pathway. These findings provide supporting evidences that genistein has promising potential as a drug for treating TBI and its subsequent neuropsychiatric detriments.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/neu.2024.22.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Data collection: Z.G. Li, Y. Li, J.K. Zhao, Z.Y. Pang, and F. Guo; design of the study: F. Guo; statistical analysis: F. Guo; analysis and interpretation of the data: Z.G. Li, Y. Li, J.K. Zhao, Z.Y. Pang, and F. Guo; drafting the manuscript: A.B. Smith, K. Baker; critical revision of the manuscript: F. Guo.
Financial support
None.
Competing interests
None.
Ethical standard
Animal studies were approved by the ethics committee of Xingtai Medical College. This study was performed in strict accordance with the National Institutes of Health guidelines for the care and use of laboratory animals (NIH Publication No. 85-23 Rev. 1985).