In response to physiological stressors, skeletal muscle has the potential to elicit a wide variety of adaptive responses, such as the biogenesis of mitochondria or clearance of damaged mitochondria to promote healthy muscle. In addition, muscle turnover can prevent metabolic imbalance, CVD and accelerated ageing(Reference Patti, Butte and Crunkhorn1,Reference Joseph, Joanisse and Baillot2) . Skeletal muscle is a highly malleable tissue, capable of considerable metabolic and morphological adaptations in response to repeated bouts of contractile activity (e.g. exercise). It is well established that chronic contractile activity, in the form of repeated bouts of endurance exercise, usually interspersed with recovery periods, results in the altered expression of a wide variety of gene products, leading to an altered muscle phenotype with improved resistance to fatigue. This improved endurance is highly correlated with increased muscle mitochondrial density and enzyme activity, referred to as mitochondrial biogenesis(Reference Nourshahi, Gholamali and Salehpour3). Exercise stimulates mitochondrial biogenesis and increases mitochondrial respiratory function and content. Several nutrients, such as polyphenols, also have an ability to induce mitochondrial biogenesis in skeletal muscle. For example, our previous study has shown that curcumin increases mitochondrial content in rat gastrocnemius muscle and that curcumin combined with endurance training additively enhances the effect of exercise on mitochondrial biogenesis(Reference Hamidie, Yamada and Ishizawa4). However, the regulatory mechanisms of nutrient-induced mitochondrial biogenesis in skeletal muscle remain unclear.
The ubiquitous second messenger cyclic AMP (cAMP) and its cellular effector, protein kinase A (PKA), constitute one of the most widely studied signalling cascades. In both mammalian cells and yeast, the regulation of mitochondrial biogenesis clearly involves the cAMP signalling pathway(Reference Yoboue, Augier and Galinier5). The phosphodiesterase (PDE) isoenzymes are essential coregulators of cAMP catabolism in many tissues, including skeletal muscle. PDE catalyses the conversion of cAMP to AMP and mediates signal termination, thereby catalysing the degradation of cAMP(Reference Conti and Beavo6). Phosphorylation of PKA activates a number of other proteins, including cAMP-response element binding protein (CREB) and liver kinase B1 (LKB-1), which increases the activity of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α)(Reference Than, Lou and Ji7,Reference Veeranki, Hwang and Sun8) . Activation of PGC-1α requires phosphorylation and deacetylation, and activated PGC-1α promotes transcription of mitochondria-related genes, which results in enhanced mitochondrial biogenesis and function(Reference Than, Lou and Ji7,Reference Veeranki, Hwang and Sun8) . Previous studies have reported that exercise, such as swimming or running, increases cAMP levels in skeletal muscle and the myocardium(Reference Palmer9) and have suggested that the mitochondrial biogenesis observed after exercise may be attributable to the increase in cAMP levels. Several polyphenols have been shown to activate cAMP and are currently under intense investigation as potential inducers of mitochondrial biogenesis(Reference Park, Ahmad and Philp10,Reference Chowanadisai, Bauerly and Tchaparian11) .
Curcumin is a polyphenol with medicinal properties found in Curcuma longa L. and turmeric, a popular culinary spice used in both vegetarian and non-vegetarian foods. The antioxidant activities of curcumin have been reported to be more potent than those of the well-studied polyphenol resveratrol(Reference Aftab and Vieira12,Reference Boarescu, Boarescu and Bocşan13) . Moreover, the long-term effects of dietary curcumin on the regulation of mitochondrial biogenesis have also been investigated(Reference Kandezi, Mohammadi and Ghaffari14). Our previous study indicated that inhibition of PKA abolished curcumin-induced phosphorylation of LKB-1, which is upstream of AMP-activated protein kinase (AMPK), suggesting that the activation of PKA may play a key role in curcumin-induced mitochondrial biogenesis(Reference Hamidie, Yamada and Ishizawa4).
Previous studies have reported that curcumin has the ability to increase mitochondrial biogenesis through an increase in cAMP levels in muscle cells(Reference Hamidie, Yamada and Ishizawa4). However, whether cAMP signalling is a critical factor for curcumin-induced mitochondrial biogenesis remains unclear. A previous study indicated that curcumin enhances β-cell function by inhibiting the expression and activity of PDE, which increases intracellular cAMP levels and subsequent insulin secretion(Reference Rouse, Younes and Egan15). In addition, administration of the phosphodiesterase 4A (PDE4A) inhibitor rolipram increases mitochondrial content and respiration by promoting the deacetylation of PGC-1α via a cAMP-dependent mechanism in myotubes(Reference Park, Ahmad and Philp16). Therefore, it is speculated that activation of cAMP signalling may be a key factor for the induction of mitochondrial biogenesis in skeletal muscle.
The present study investigated (1) whether cAMP and PKA signalling is involved in curcumin-induced enhancement of mitochondrial biogenesis and respiration, and (2) whether the regulatory mechanism of curcumin-induced mitochondrial biogenesis is different from that of exercise-induced mechanisms in rat gastrocnemius muscle.
Material and methods
Animal treatments
The Ethics Committee on Animal Experimentation of Kanazawa University approved all procedures performed in the present study (Protocol: AP-10187). Two experimental designs were used in the present study. One study determined the effect of curcumin on skeletal muscle mitochondrial biogenesis and respiration (results shown in Figs. 1–3). The second study clarified the possible underlying mechanisms by focusing on PDE and PKA signalling with or without exercise (results shown in Figs. 4–7). Eighteen male Wistar rats (body weight (BW) 190–205 g), aged 8 weeks, were used (nine rats per group) to determine the effect of curcumin on mitochondrial biogenesis and respiration. The animals were kept in an air-conditioned room and exposed to a 12-h light–dark photoperiod. Two rats were housed per cage and were provided with a standard diet (Oriental Yeast, Tokyo, Japan) and water ad libitum. The rats were randomly assigned to control and curcumin (CURC) groups. All animals received daily intraperitoneal (i.p.) injections with curcumin (100 mg/kg-BW/d) dissolved in dimethyl sulfoxide (DMSO) or the same volume of DMSO (5 ml/kg-BW) for 28 d.
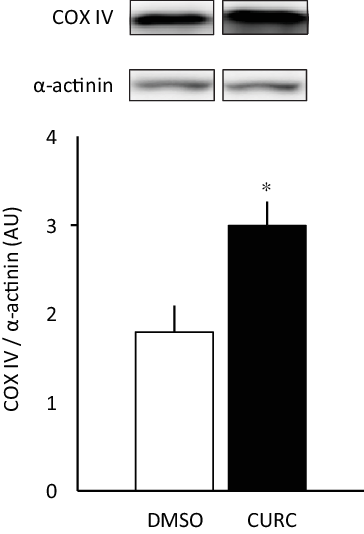
Fig. 1. Curcumin treatment increases COX-IV protein expression. Curcumin (CURC) treatment (100 mg/kg-BW/d for 28 d) increased COX-IV protein expression in gastrocnemius skeletal muscle. Values are presented as mean ± sd (n 6 in each group). *: significantly different from the DMSO group (P < 0·05). COX-IV, cytochrome c oxidase subunit; DMSO, dimethyl sulfoxide; BW, body weight.
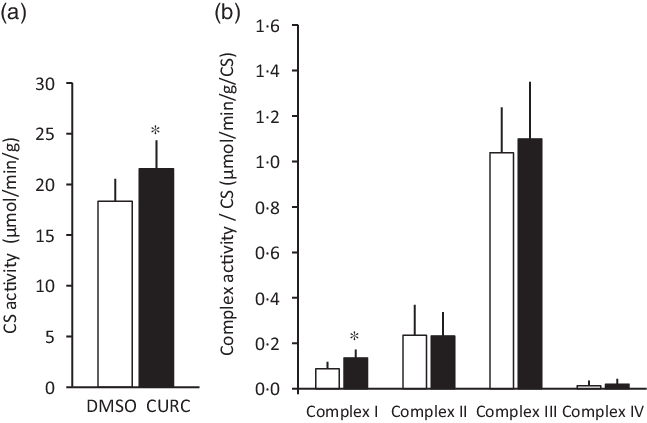
Fig. 2. Curcumin treatment increases CS activity and respiratory complex enzymatic activity. Curcumin treatment increased CS activity (a) and respiratory complex I enzymatic activity (b) in skeletal muscle. Values are presented as mean ± sd (n 9 in each group). *: significantly different from the DMSO group (P < 0·05). CS: citrate synthase, CURC: curcumin (100 mg/kg-BW/d for 28 d). ![]() , DMSO;
, DMSO; ![]() , CURC. DMSO, dimethyl sulfoxide; BW, body weight.
, CURC. DMSO, dimethyl sulfoxide; BW, body weight.
In the second study, twenty-four 8-week-old male Wistar rats (six rats per group) were randomly assigned to two primary groups: exercise and non-exercise. Subsequently, each group was subdivided into control, curcumin (CURC), H89 (a PKA inhibitor) and CURC + H89 groups to determine the involvement of PKA signalling in curcumin and/or exercise-induced mitochondrial biogenesis. Rats in the three treatment groups were injected i.p. with curcumin (100 mg/kg-BW/d) and/or H89 (20 mg/kg-BW/d) dissolved in DMSO, while control rats received the same volume of DMSO once a day for 3 d. To further determine the involvement of PDE signalling, injection of the selective PDE4 inhibitor rolipram (10 mg/kg-BW/d, i.p., once a day for 3 d) was also conducted. The exercise training group rats swam 2 h/d in four 30-min sessions separated by 5 min of rest. After the first 30-min bout, a weight equal to 2 % of the animal’s BW was tied to the body of each rat using a plastic cable tie. The rats swam with the weight attached for the remaining three exercise bouts. All rats swam in a barrel filled to a depth of 50 cm with a swimming area of 190 cm2/rat(Reference Kawa, naka, Tabata and Katsuta17). The rats performed the above swimming protocol once a day for 28 and 3 d.
Isolation of muscle fibres and permeabilisation
Calf muscles were isolated under anaesthesia with i.p. injection of medetomidine (0·3 mg/kg), midazolam (4 mg/kg) and butorphanol (5 mg/kg). Permeabilisation was conducted according to a previously reported method(Reference Kuznetsov, Veksler and Gellerich18). Fresh muscle samples were divided into small pieces (200–250 mg) in isolation buffer (2·77 mM CaK2EGTA, 7·23 mM K2EGTA, 20 mM imidazole, 20 mM taurine, 49 mM K-MES, 3 mM K2HPO4, 9·5 mM MgCl2, 5·7 mM ATP, 15 mM phosphocreatine and 1 µM leupeptin, pH 7·1). Under a microscope, the muscle fibres were isolated from the muscle specimens in the isolation buffer with sharp forceps to avoid any mechanical stress. The isolated muscle fibres were incubated in the isolation buffer with 0·75 mg/ml collagenase for 30 min(Reference Toleikis, Majiene and Trumbeckaite19). Afterwards, the muscle fibres were incubated in the buffer with 100 µg/ml saponin for 30 min. Lastly, the fibres were washed for 10 min with respiration buffer (0·5 mM EGTA, 3 mM MgCl2–6H2O, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES-KOH, 1 g/l BSA, 60 mM potassium lactobionate, 110 mM mannitol and 0·3 mM dithiothreitol, pH 7·1) and stored in the same buffer until measurement of mitochondrial respiration.
Mitochondrial respiration
Measurements of mitochondrial respiration were performed as described previously(Reference Kuznetsov, Veksler and Gellerich18). Mitochondrial oxygen consumption was monitored using an Oxygraph-2 k (Oroboros, Innsbruck, Austria) at 37 °C in a 2-ml thermostatically controlled chamber equipped with polarographic oxygen sensors in the respiration buffer. This buffer was sterilised by filtration through a 0·45-µm filter. Malate (2 mM) and glutamate (10 mM) were then added to induce state 4 respiration, which is dependent on complexes I, III and IV. Thereafter, ADP (5 mM) was added to the chambers to induce state 3 respiration. Exactly 10 mM succinate was added to activate respiration via complexes II, III and IV in the former state 3 respiration (state 3 + succinate). Next, 1 µm oligomycin was added to block complex V and the residual respiration is due to proton leakage (H+ leak).
Differences in the mitochondrial respiration between the state 3 + succinate condition and the H+ leak condition are regarded as coupling respiration (ATP turnover = [state 3 + succinate] – [H+ leak]). Cytochrome c oxidase subunit IV (COX-IV)-dependent maximal VO2 was monitored after the addition of 5 mM of TMPD (N,N,N',N'-tetramethyl-p-phenylenediamine) and 5 mM of ascorbate. TMPD was added after addition of ascorbate to the chamber to avoid oxidation. Finally, 2·5 µM antimycin A, a complex III inhibitor, was added to the chambers to stop respiration. Under this situation, any residual respiration is non-mitochondrial and was subtracted from the respiration rates obtained under the other state.
Based on the above measurements of the mitochondrial respiration states, the respiratory control ratio (RCR = [state 3]/[state 4]) and the coupling ratio (CR = [state 3 + succinate]/[H+ leak]) were calculated.
Mitochondrial enzymatic activity
A small sample of skeletal muscle (approx. 20 mg) was homogenised on ice in 0·1 M TRIS buffer containing 0·1 % Triton X-100, pH 8·0. Citrate synthase (CS) activity was determined spectrophotometrically according to Srere’s method(Reference Srere and Lowenstein20). The homogenates were frozen and thawed three times to disrupt the mitochondria and expose CS. The assay was performed in a total volume of 900 µl: 100 mM TRIS buffer (pH 8·0), 1 mM 5,5-dithiobis (2-nitrobenzoate) (DTNB), 3 mM acetyl-CoA, 5 mM oxaloacetate (OAA) and 100 µl of muscle homogenate. The principle of the assay is to initiate the reaction of acetyl-CoA with OAA and link the release of free CoA-SH to the colorimetric reagent DTNB. All measurements were performed in duplicate at 30 °C. CS activity was normalised to total protein content and expressed as micromoles per gram protein per minute.
Mitochondrial respiratory chain complex activities were determined based on a spectrophotometric assessment, as previously described(Reference Spinazzi, Casarin and Pertegato21). Subsequently, the ubiquinone analogue decylubiquinone (10 mM), as an electron acceptor, was utilised to determine oxidation of NADH (10 mM) by complex I (NADH–ubiquinone reductase). Oxidation of succinate (400 mM) by complex II (FADH2–ubiquinone reductase) was determined with decylubiquinone as an electron acceptor. Oxidised cytochrome c, as an electron acceptor, was used to determine oxidation of decylubiquinol (10 mM) by complex III (ubiquinol–cytochrome c reductase). The reduced cytochrome c, as a substrate with O2 (dissolved oxygen in cuvettes) as an acceptor, was exposed to determine complex IV (cytochrome c oxidase)(Reference Rossignol, Malgat and Mazat22).
Nuclear fraction preparation
Animals were anesthetised with medetomidine (0·3 mg/kg), midazolam (4 mg/kg) and butorphanol (5 mg/kg) 2 hours after the last endurance exercise session. The gastrocnemius muscle was then isolated for biochemical analysis. The tissues were washed in ice-cold saline and cleaned of connective tissue and nerves. The tissue samples were then frozen rapidly in liquid N2. Nuclear fractions were isolated using a modified version of the protocol established by Blough(Reference Blough, Dineen and Esser23) and divided into 1-ml PBS aliquots.
Immunoprecipitation and western blotting
Lysis buffer (20 mM TRIS-HCl [pH 7·4], 50 mM NaCl, 250 mM sucrose, 50 mM NaF, 5 mM sodium pyrophosphate, 1 mM Dithiothreitol (DTT), 4 mg/l leupeptin, 50 mg/l trypsin inhibitor, 0·1 mM benzamidine and 0·5 mM PMSF) was used for immunoprecipitation (IP). Dynabeads (Invitrogen) were added to the nuclear protein fractions and incubated at 4 °C for 1 h. The Dynabeads were then collected using magnets before addition of normal mouse Ig G (nIgG; Santa Cruz Biotechnology) to the supernatants and incubated at 4 °C overnight. The samples were centrifuged at 7000 g for 30 s at 4 °C, and aliquots of this supernatant were incubated with primary antibodies against PGC-1α and mouse nIgG at 4 °C overnight. Dynabeads were then added and incubated at 4 °C for 2 h. The pellets were collected using magnets and subsequently washed with PBS. The final pellet from the IP was collected and subjected to western blotting analysis as previously described(Reference Furuichi, Sugiura and Kato24). Briefly, equal amounts of protein samples were loaded into SDS-PAGE gels: 12·5 % (COX-IV), 10 % (phospho-PDE4A, PDE4A, phospho-AMPKα and AMPKα), or 7·5 % (PGC-1α and acetylated lysine). The proteins were transferred onto a polyvinylidene fluoride membrane that was incubated in blocking buffer and subsequently incubated with the following primary antibodies: phospho-PDE4A (phospho S) (1:500 dilution; Abcam, ab14610), PDE4A (1:500 dilution; Santa Cruz Biotechnology, sc-25811), Phospho-AMPKα (Thr 172) (1:1000 dilution; Cell Signaling Technology, #2535), AMPKα (1:1000 dilution; Cell Signaling Technology, #2603), PGC-1α (1:500 dilution; Calbiochem, ST1202), acetylated–lysine (1:1000 dilution; Cell Signaling Technology, #9441), COX-IV (1:1000 dilution; Abcam, ab14744), α-actinin (1:1000 dilution; Cell Signaling Technology, #3134), β-actin (1:1000 dilution; Abcam, ab8226) and lamin (1:1000 dilution; Santa Cruz Biotechnology, sc-20681). Finally, protein bands were detected with ECL Prime reagents (GE Healthcare) using a digital capture system (MicroChemi, DNR Bio-imaging Systems, Ltd), and the signal intensities were quantified using Image J (version 1.46, NIH).
Determination of cyclic AMP levels
The cAMP Direct Immunoassay Kit from Abcam (ab65355) was used to measure the levels of cAMP in cell extracts. cAMP quantitation was carried out in accordance with the manufacturer’s instructions. Briefly, 50 μl of the acetylated standard cAMP and test samples were added to a protein G-coated 96-well plate. This step was followed by the addition of 10 μl of reconstituted cAMP antibody per well. The samples were then incubated for 1 h at room temperature with gentle agitation and subsequently washed five times with 200 μl 1 × Assay Buffer. The wells were completely emptied by tapping the plate on a clean paper towel after each wash. After confirming that there was no liquid remaining, 100 μl of HRP developer was added and incubated for 1 h at room temperature with agitation. Finally, the reaction was stopped by adding 100 μl of 1 M HCl to each well (sample colour changed from blue to yellow), and the plate was read at OD450 nm by spectrophotometry (Infinite 200 PRO).
Statistical analysis
The unpaired Student’s t test was used to assess differences between DMSO and CURC conditions in mitochondrial biogenesis, respiration and enzymatic activity. Two-way ANOVA was used to assess the effect of exercise and inhibitors together with curcumin on cAMP levels, phosphorylation of PDE4A and AMPK, and acetylated PGC-1α expression. The Tukey–Kramer post hoc test was used to assess differences between experimental groups. All data are expressed as mean ± standard deviation (sd). Values of P < 0·05 were considered significant.
Results
Food intake and physical characteristics of the animals
No significant difference in food intake was observed between the control (DMSO) and CURC group. Curcumin treatment also had no effect on hindlimb muscle weight. However, BW and abdominal fat mass were significantly lower in the CURC group than in the control group (Table 1).
Table 1. Food consumption and physical characteristics of animals
(Mean values and standard deviation, n 9 in each group)
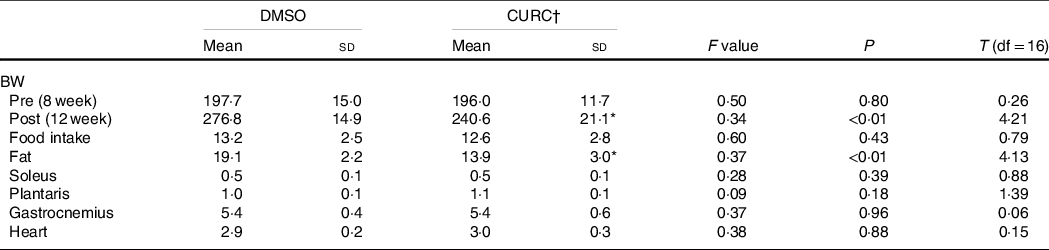
DMSO, dimethyl sulfoxide; CURC, curcumin; BW, body weight.
* Significantly different with DMSO group (P < 0.05).
† All units: gram. CURC: curcumin (100 mg/kg-BW/d).
Effect of CURC on mitochondrial protein levels, enzymatic activities and respiration in muscle
The 28-d curcumin treatment resulted in a significant increase in mitochondrial protein expression (COX-IV) and CS activity in gastrocnemius muscle tissue (Figs. 1 and 2(a)). This suggests that curcumin administration induced mitochondrial biogenesis. Mitochondrial respiratory complex activities were normalised by CS activity, and it was found that curcumin treatment increased the activity respiratory complex I (Fig. 2(b)), but not complexes II, III and IV (Fig. 2(b)). No significant differences in CS-normalised mitochondrial respiratory capacity were observed between CURC and control (DMSO) animals at any state (Fig. 3(a)). Moreover, no significant differences in the RCR and CR ratios were observed (Fig. 3(b)).
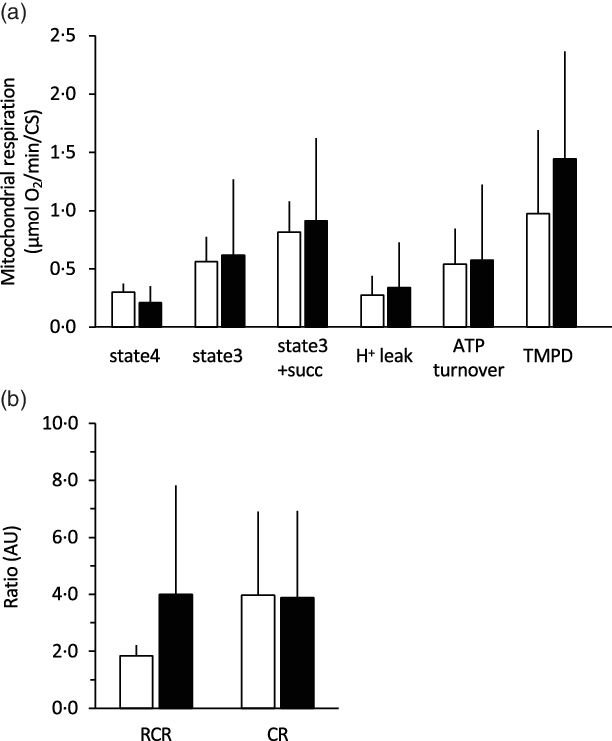
Fig. 3. Effect of curcumin treatment on mitochondrial respiratory capacity. For all mitochondrial states, curcumin treatment did not increase mitochondrial VO2 (a), RCR or CR ratio (b). Mitochondrial respiration was normalised to CS activity. RCR: respiratory control ratio. CR: coupling ratio. Values are presented as mean ± sd (n 9 in each group). CS: citrate synthase, CURC: curcumin (100 mg/kg-BW/d for 28 d). ![]() , DMSO;
, DMSO; ![]() , CURC. RCR, respiratory control ratio; CR, coupling ratio; BW, body weight.
, CURC. RCR, respiratory control ratio; CR, coupling ratio; BW, body weight.
Curcumin treatment has an effect on cyclic AMP similar to that of rolipram
Analysis of cAMP levels with two-way ANOVA indicated significant main effects (treatment and exercise) without a significant interaction effect (Fig. 4). Compared with the DMSO group, gastrocnemius muscle cAMP levels in the CURC and rolipram groups were significantly greater. The cAMP levels were also significantly higher in animals given exercise than in non-exercise animals. When considering the exercise condition, although not statistically significant, cAMP levels in the CURC and rolipram groups tended to be 32 and 26 % higher than in the DMSO group, respectively. These results suggest that curcumin produces an effect on skeletal muscle cAMP levels that is similar to rolipram.
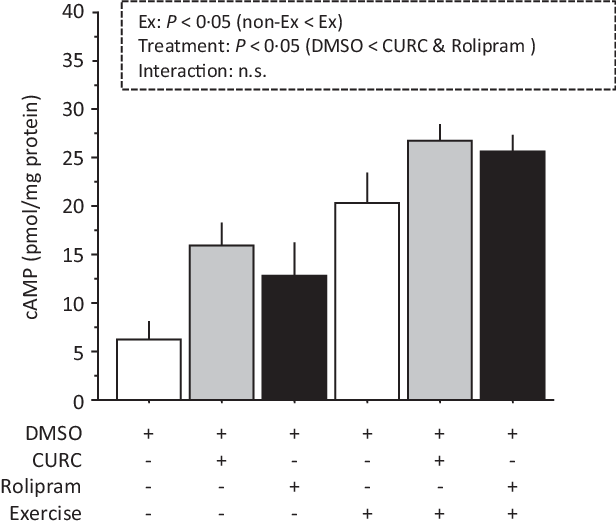
Fig. 4. Curcumin and rolipram produce similar effects on cAMP levels in gastrocnemius muscle. Two-way ANOVA of cAMP levels in gastrocnemius muscle revealed significant main effects for exercise (P < 0·05) and curcumin treatment (P < 0·05) with no significant interaction effect. Values are presented as mean ± sd (n 6 in each group). DMSO = control. CURC = curcumin 100 mg/kg-BW/d in DMSO for 28 d. Rolipram = rolipram 10 mg/kg-BW/d in DMSO. Ex = exercise. DMSO, dimethyl sulfoxide; BW, body weight; cAMP, cyclic AMP.
Curcumin treatment inhibits phosphodiesterase 4A phosphorylation
Two-way ANOVA of phospho-PDE4A levels revealed significant main effects (treatment and exercise) with a significant interaction (Fig. 5). Exercise promoted PDE4A phosphorylation in the gastrocnemius muscle, whereas curcumin treatment significantly attenuated PDE4A phosphorylation regardless of the presence of exercise. These results suggest that curcumin inhibits PDE4A phosphorylation in skeletal muscle.
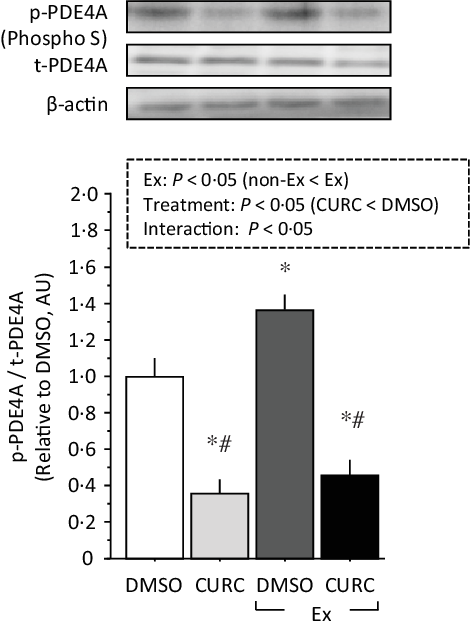
Fig. 5. Curcumin treatment inhibits phosphorylation of PDE4A in gastrocnemius muscle. Two-way ANOVA of PDE4A phosphorylation in gastrocnemius muscle revealed significant main effects for exercise (P < 0·05) and curcumin treatment (P < 0·05) with a significant interaction effect (P < 0·05). Values are presented as mean ± sd (n 6 in each group). DMSO = control. CURC = curcumin 100 mg/kg-BW/d in DMSO for 28 d. *: significantly different from DMSO without exercise group (P < 0·05). #: significantly different from DMSO + exercise group (P < 0·05). DMSO, dimethyl sulfoxide; BW, body weight; PDE4A, phosphodiesterase 4A.
Protein kinase A inhibition reduces the effect of curcumin on AMP-activated protein kinase phosphorylation, but not deacetylation of peroxisome proliferator-activated receptor gamma coactivator-1α
PGC-1α activation via PKA/AMPK signalling plays a key role in the induction of mitochondrial biogenesis in skeletal muscle(Reference Fernandez-Marcos and Auwerx25). Therefore, we focused on PKA and its downstream targets AMPK and PGC-1α in the present study. Analysis of AMPK phosphorylation with two-way ANOVA indicated significant main effects (exercise and treatment) with a significant interaction (Fig. 6). Curcumin alone and exercise (with or without curcumin) significantly increased the phosphorylation of AMPK. However, H89 treatment combined with curcumin and/or exercise significantly reduced AMPK phosphorylation, completely inhibiting the effects of curcumin and/or exercise.
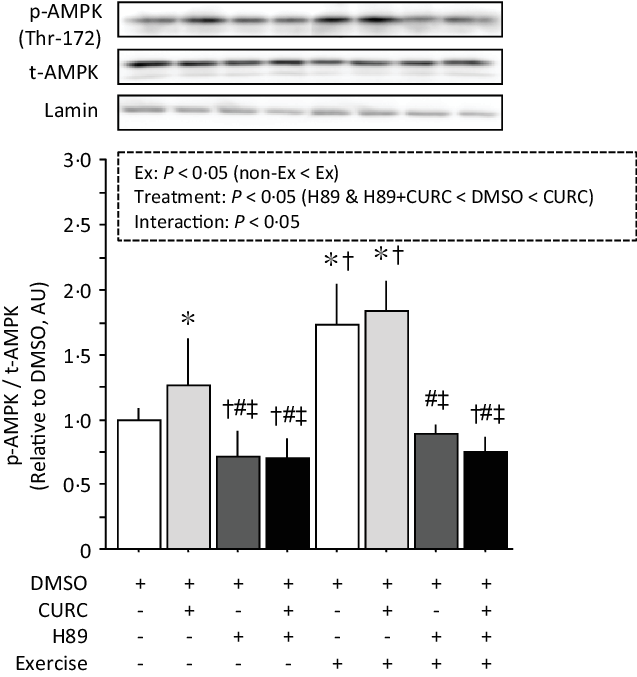
Fig. 6. Curcumin-induced phosphorylation of AMPK (Thr-172) in gastrocnemius muscle is abolished by H89. Two-way ANOVA of AMPK phosphorylation in gastrocnemius muscle revealed significant main effects for exercise (P < 0·05) and curcumin treatment (P < 0·05) with a significant interaction effect (P < 0·05). Values are presented as mean ± sd (n 6 in each group). DMSO = control without exercise. CURC = curcumin 100 mg/kg-BW/d in DMSO for 28 days. H89 = PKA inhibitor 20 mg/kg-BW/d. Ex = exercise. *: significantly different from DMSO without exercise group (P < 0·05). †: significantly different from curcumin without exercise (P < 0·05). #: significantly different from DMSO + exercise (P < 0·05). ‡: significantly different from curcumin + exercise. AMPK, AMP-activated protein kinase; DMSO, dimethyl sulfoxide; BW, body weight; PKA, protein kinase A.
Analysis of the acetylation of PGC-1α with two-way ANOVA revealed significant main effects (treatment and exercise) without a significant interaction effect (Fig. 7). Acetylated PGC-1α levels were significantly lower in CURC than in DMSO, H89 and H89 + CURC animals. In addition, acetylated PGC-1α levels in the exercise group were also significantly lower than in the non-exercise group.
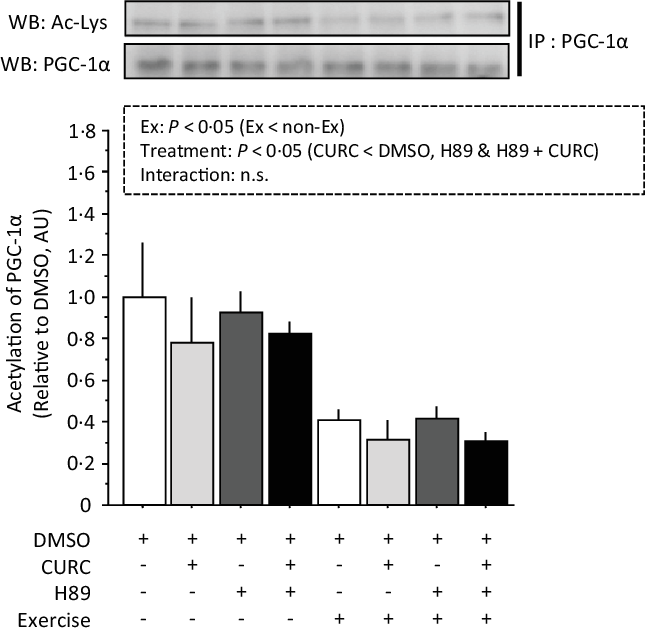
Fig. 7. Exercise-induced decreases in PGC-1α acetylation in gastrocnemius muscle are abolished by H89. Two-way ANOVA of PGC-1α acetylation in gastrocnemius muscle revealed significant main effects for exercise (P < 0·05) and curcumin treatment (P < 0·05) with no significant interaction effect (n.s.). Values are presented as mean ± sd (n 6 in each group). DMSO = control without exercise. CURC = curcumin 100 mg/kg-BW/d in DMSO for 28 d. H89 = PKA inhibitor 20 mg/kg-BW/d. Ex = exercise. DMSO, dimethyl sulfoxide; BW, body weight; PKA, protein kinase A; PGC-1α, PPAR gamma coactivator.
These results indicate that PKA plays a key role in curcumin-induced AMPK phosphorylation, and that curcumin is not directly related to the subsequent activation of PGC-1α.
Discussion
Previous studies in this field determined that certain dietary compounds, such as curcumin, produce beneficial effects for the prevention or treatment of obesity and metabolic disorder(Reference Akbari, Lankarani and Tabrizi26). In agreement with these findings, the present study indicates that 28 d of curcumin administration (100 mg/kg-BW/d) has the ability to decrease abdominal fat mass and results in reduced BW (Table 1). This result may support the idea that curcumin has the potential to improve obesity and its related metabolic disorders.
Consistent with previous observations(Reference Hamidie, Yamada and Ishizawa4), the present study showed that a 28-d treatment with the polyphenol curcumin increased COX-IV protein expression, as well as CS activity, in skeletal muscle (Figs. 1 and 2(a)). The enzyme cytochrome c oxidase (complex IV) is a large transmembrane protein complex present in mitochondria. It is the last enzyme in the respiratory electron transport chain and is located in the inner membrane. A previous study that used electrical stimulation to increase COX-IV activity showed evidence for a correlation between PGC-1α and mitochondrial biogenesis(Reference Irrcher, Adhihetty and Sheehan27). Several polyphenols have the ability to increase PGC-1α and sirtuin-1 (SIRT1) protein expression in skeletal muscle, which leads to stimulation of mitochondrial biogenesis(Reference Davis, Murphy and Carmichael28). Indeed, our previous study also showed that curcumin has the ability to stimulate mitochondrial biogenesis and COX-IV in skeletal muscle through the AMPK-SIRT1-PGC-1α pathway, and that this phenomenon is enhanced in combination with endurance training(Reference Hamidie, Yamada and Ishizawa4). Our findings emphasised that curcumin induces mitochondrial biogenesis in skeletal muscle and has additive effects with exercise, as represented by mitochondrial protein expression analysis.
Furthermore, in the present study, we assessed the effects of curcumin on the respiratory capacity of skeletal muscle mitochondria. Although the previous results indicated that curcumin increases mitochondrial biogenesis(Reference Hamidie, Yamada and Ishizawa4), mitochondrial VO2 was not directly evaluated. Thus, the present study examined mitochondrial respiration in permeabilised muscle fibres to determine if mitochondrial respiratory function was negatively impacted by curcumin. The results in Fig. 3 suggest that curcumin treatment did not negatively impact mitochondrial respiration. On the other hand, curcumin treatment was observed to significantly increase the activity of certain respiratory enzymes, specifically complex I, but not complexes II, III and IV (Fig. 2). A previous study suggested that complex I is the key entry point for electrons to the respiratory chain and plays a central role in energy metabolism(Reference Sharma, Lu and Bai29). Thus, the present results potentially indicate an underlying mechanism by which curcumin treatment increases intact mitochondria in skeletal muscle. Our results are also supported by the observed ability of curcumin to restore mitochondrial respiration and increase ATPase activity in diabetic mice(Reference Soto-Urquieta, Lope, z-Briones and Perez-Vazquez30). Future studies aimed at examining interactions between curcumin and complex I will further evaluate the ability of curcumin to increase mitochondrial respiratory capacity.
Previous studies have shown that forskolin (an adenylyl cyclase activator) induces cAMP(Reference Abukhashim, Wiebe and Seubert31). Elevated cAMP levels subsequently activate PDE, which catalyses degradation of cAMP to AMP(Reference Boularan and Gales32). Conversely, inhibition of PDE with rolipram results in increased cAMP levels(Reference Lelkes, Alföldi and Erdos33). Consistent with expectations, our data indicate that rolipram, as well as curcumin, increases cAMP levels in gastrocnemius muscle (Fig. 4). Previous studies also show that curcumin inhibits PDE in endothelial cells(Reference Abusnina, Keravis and Lugnier34), pancreatic β-cells(Reference Rouse, Younes and Egan15) and endothelial cells(Reference Abusnina, Keravis and Zhou35). Furthermore, the polyphenol resveratrol causes an increase in cAMP concentrations, not by increasing cAMP production but by inhibiting cAMP PDE(Reference Chung, Manganiello and Dyck36). These findings suggest that curcumin increases cAMP levels via the inhibition of PDE signalling in skeletal muscle. Interestingly, the present study also showed that curcumin and exercise, as well as rolipram and exercise, tended to produce additive effects on cAMP levels in skeletal muscle (Fig. 4). Furthermore, the extent to which curcumin inhibited PDE4A phosphorylation was similar in the absence and presence of exercise, even though exercise itself increased the phosphorylation of PDE4A (Fig. 5). These observations indicate that curcumin strongly inhibits PDE4A and thereby stimulates cAMP expression in skeletal muscle; a mechanism that is potentially distinct from the exercise-induced mechanism.
The most common downstream effector of cAMP is PKA(Reference Taskén and Aandahl37). When a molecule of PKA binds to four molecules of cAMP, the PKA molecule releases two subunits that subsequently exert enzymatic activity on target proteins(Reference Skalhegg and Tasken38), including phosphorylation of LKB-1 and CREB. The previous investigation of the effects of curcumin on gene expression indicated that PKA is an important regulator of cellular responses to curcumin(Reference Swatson, Katoh-Kurasawa and Shaulsky39). Furthermore, our previous report stated that curcumin increased the levels of downstream targets of PKA, namely phosphorylation of LKB-1 and CREB in gastrocnemius(Reference Hamidie, Yamada and Ishizawa4). These results are supported by previous studies of the effects of curcumin administration on diabetic rats, where curcumin increased LKB-1 phosphorylation in skeletal muscle(Reference Na, Zhang and Li40) and CREB phosphorylation in cerebral cortex(Reference Kumar, Antony and Gireesh41). LKB-1 is upstream relative to AMPK, which can act as an energy sensor in cells and functions as a key regulator of mitochondrial biogenesis via deacetylation of its downstream target PGC-1α. It has been also reported that PKA is involved in increasing LKB-1 phosphorylation(Reference Collins, Reoma and Gamm42). These findings suggest that the activation of the cAMP/PKA/LKB-1 pathway followed by phosphorylation of AMPK and deacetylation of PGC-1α has an important role in the induction of mitochondrial biogenesis in skeletal muscle. Therefore, we evaluated the effect of curcumin on AMPK phosphorylation and PGC-1α deacetylation in skeletal muscle. We demonstrated that AMPK phosphorylation was stimulated by curcumin in both the absence and presence of exercise (Fig. 6). In addition, the individual and combined effects of curcumin and/or endurance exercise on AMPK phosphorylation were completely abolished by treatment with the PKA inhibitor H89. These results were partially supported by our previous finding, which indicated that H89 treatment suppressed curcumin- and/or exercise-induced promotion of LKB-1 phosphorylation(Reference Hamidie, Yamada and Ishizawa4). Therefore, AMPK activation via the cAMP/PKA pathway is thought to be important for the induction of mitochondrial biogenesis by curcumin treatment in skeletal muscle. Our data also suggest curcumin and endurance exercise may partially share a mechanism for AMPK activation, whereby curcumin and exercise produce additive effects on AMPK phosphorylation.
Although curcumin and/or exercise promoted PGC-1α deacetylation in the present study, the impact of PKA inhibition on PGC-1α deacetylation was less than the observed effects on AMPK phosphorylation (Figs. 6 and 7). This observation indicates that PGC-1α deacetylation may not be a main target of activated AMPK in curcumin- and/or endurance exercise-induced mitochondrial biogenesis in skeletal muscle. It has been reported that activated AMPK is involved in PGC-1α deacetylation via SIRT1 activation as well as direct phosphorylation of PGC-1α. Jäger(Reference Jäger, Handschin and St.-Pierre43) reported that AMPK-mediated phosphorylation of PGC-1α activates PGC-1α and stimulates mitochondrial biogenesis in skeletal muscle. In addition, activation of CREB also promotes PGC-1α-mediated mitochondrial biogenesis(Reference Fernandez-Marcos and Auwerx44). Furthermore, we have previously shown that treatment with H89 inhibited the expression levels of SIRT1 and CREB in rat gastrocnemius muscle(Reference Hamidie, Yamada and Ishizawa4). Collectively, all aforementioned findings suggest that curcumin- and/or exercise-induced mitochondrial biogenesis in the present study may be due in part to the activation of PKA/AMPK/PGC-1α phosphorylation and/or the PKA/CREB pathway. Further studies are required to clarify these mechanisms.
Our present and previous data indicated that i.p. curcumin administration at a dose of 100 mg/BW alone increased mitochondrial content in rat skeletal muscle, and this effect was additively enhanced when combined with endurance exercise(Reference Hamidie, Yamada and Ishizawa4). However, the magnitude of increased mitochondrial content was less in the curcumin treatment than in the exercise condition(Reference Hamidie, Yamada and Ishizawa4). Unfortunately, there is limited information regarding the health benefits of dietary curcumin supplementation on skeletal muscle. Receno reported that daily curcumin ingestion (0·2 % of food intake) combined with reduced food intake for 4 months improved skeletal muscle mass and function in aged rats(Reference Receno, Liang and Korol45). Tanabe also showed that short-term (7 d) oral supplementation of 90 mg curcumin twice daily (total: 180 mg/d) after eccentric exercise attenuated muscle damage and facilitated recovery following muscle damage in humans(Reference Tanabe, Chino and Ohnishi46). Therefore, these observations suggest that dietary curcumin supplementation cannot replace exercise but has the potential to additively enhance the health benefits of exercise and its effect on skeletal muscle.
Conclusion
The present study concludes that i.p. curcumin treatment induces mitochondrial biogenesis through increased cAMP levels attributable to inhibition of PDE4A and subsequent activation of the PKA/LKB-1/AMPK pathway in skeletal muscle. We also found that curcumin and endurance exercise act as an inhibitor or enhancer of PDE4A, respectively, although both treatments result in elevation of cAMP levels. These findings suggest that the mechanism underlying the ability of curcumin to stimulate mitochondrial biogenesis in skeletal muscle is distinct from the exercise-induced mechanism.
Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture for K. Masuda (no. 15K12666 and no. 17H02158), and also by funding from Shibuya Science, Culture and Sport Foundation, Japan for K. Masuda. R.D.R. Hamidie was supported by Directorate General of High Education, Indonesia, for the international collaboration fund scheme Universitas Pendidikan Indonesia (no. 339/UN.40D/PP/2018).
H.R. and K.M. conceptualized the project and design of the research; H.R., T.H. and T.Y. performed the experiments; H.R., T.S., T.H., R.K., and K.M. analyzed the data; H.R., T.S., T.Y., R.I., Y.S., and K.M. curated data of the experiment; H.R., T.S., T.H., R.K. and K.M. prepared visualization; H.R. and K.M. wrote the original manuscript; H.R., T.S., R.K., Y.S. and K.M. reviewed and edited the manuscript. All authors approved the final version of the manuscript.
The authors declare that have no competing interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114521000490











