Early weaning in infant mammals usually co-occurs with severe stress due to nutritional, physiological, environmental, social and psychological transitions. In the pig industry, piglets undergoing early weaning are often characterised by reduced growth and increased prevalence of diarrhoea after weaning; the latter is commonly considered as major health issue, as it always leads to substantial morbidity and mortality in piglets and results in huge economic losses in pig production(Reference Lauridsen1). Although antibiotics and zinc oxide can effectively treat diarrhoea during the weaning process, the shortcomings of these measures also give rise to concerns regarding public health and environmental pollution(Reference Gao, Yin and Xu2). Thus, there is an urgent need to identify effective alternatives to reduce or alleviate weaning-associated syndromes.
Intestinal microbiome undergoes dramatic changes from birth to weaning in piglets(Reference Guevarra, Lee and Lee3), and accumulated data highlight the contribution of gut microbiota dysbiosis as a leading cause of post-weaning growth retardation or diarrhoea(Reference Gresse, Chaucheyras-Durand and Fleury4). Meanwhile, the diet and environmental stimuli in early life exert a significant intervention on the development of the gut microbiota in piglets. Direct evidence suggests that maternal administration of soluble fibre(Reference Cheng, Wei and Xu5) and resveratrol(Reference Meng, Sun and Luo6) during pregnancy and/or lactation can improve the resistance to diarrhoea or diseases via reshaping the composition of the intestinal or fecal microbiota, while heat stress during late gestation can alter offspring’s gut microbial colonisation(Reference He, Zheng and Tao7). Herein, it is a promising strategy to prevent or alleviate weaning stress and post-weaning diarrhoea by optimising maternal nutrition during gestation and/or lactation.
ω-3 PUFA consist of EPA, DHA, α-linolenic acid as well as DPA, an intermediate between EPA and DHA. Distinct from short- or medium-chain fatty acids which can serve as direct energy sources, essential ω-3 PUFA are always incorporated into cell membranes and thereby exert biological functions in relation to health and immunity(Reference Lauridsen1). Recent reports also indicate that ω-3 PUFA also participate in the maintenance of gut homeostasis, which is associated with gut microbiota and intestinal health(Reference Fu, Wang and Gao8). Furthermore, maternal ω-3 PUFA have been illustrated to transfer to offsprings via milk and provide multifarious benefits to the offspring, including better cognitive performance(Reference Lehner, Staub and Aldakak9), alleviation of inflammation(Reference Haghiac, Yang and Presley10) or enhanced immunity(Reference Myles, Pincus and Fontecilla11,Reference Jolazadeh, Mohammadabadi and Dehghan-Banadaky12) by remodelling the gut microbiota community. The emerging effects of maternal fish oil on the growth performance, immune activity of nursery piglets(Reference You, Lee and Oh13,Reference Chen, Xu and Li14) encourage us to further verify whether ω-3 PUFA can effectively alleviate post-weaning diarrhoea and improve gut health in piglets.
In present study, ω-3 PUFA-rich fish oil was administrated from late gestation to lactation, and growth performance, immune activity, post-weaning diarrhoea and gut microbiota community in piglets were measured. The results are relevant to seeking potential strategies for improving the adaptation of piglets during the suckling-to-weaning transition.
Materials and methods
Animal management and sampling procedures were approved by the Animal Care and Use Committee of Northwest A&F University and were in accordance with the National Research Council’s Guideline for the Care and use of Laboratory Animals.
Experimental scheme and animal care
This experiment was carried out at the Hengzhuang pig farm. A total of forty-five Landrace × Yorkshire gestating sows (85 d of gestation, parity = 1, bodyweight 212·35 ± 1·61 kg, mean ± se) were randomly allocated to one of three dietary treatments according to their body weight and backfat thickness, with fifteen replicates per group. The dietary treatment comprised a basal commercial gestation or lactation diet (control, CON group; as listed in Supplementary Table S1) or the same basal diet supplemented with 30 or 60 g/d fish oil per sow from day 90 of gestation to weaning at day 21 of lactation, respectively, after 5 d of a pre-feeding period. Fish oil were offered to sows individually before feed was supplied in the morning, and sows generally finished the aliquots of fish oil within 5 min. Fish oil was obtained from Bailushi BioTech Ltd. Company. The concentrations of EPA and DHA were determined by GC-MS with the help of Panomix, and results shown that fish oil was comprised of 13·33 % DHA and 5·40 % EPA, and the ratio of EPA to DHA was 1:2·47.
Sows were housed in individual farrowing stalls (2·1 m × 0·65 m) during the gestational period from day 85 to day 110. From day 100 of gestation to ablactation, the sows were transferred into 2·2 m × 1·8 m concrete-floored delivery pens. The day of parturition was defined as day 0 of lactation and the piglets were weaned at day 21 of lactation. Sows were fed twice a day (08:00 am and 14:00 pm) from late gestation to the first week of lactation and were fed ad libitum throughout lactation. According to the number of effective teats of sows, litters were standardised to ∼12 piglets per sow within 24 h after birth by cross-fostering within treatments. Piglets were weighed individually on the 1st and 21th day of age. Nursing piglets got access to creep feed from 7 d after birth to the end of experiment (28 d after birth, or 1 week post-weaning), and the ingredients of creep feed were listed in Supplementary Table S2. All feeds in this study were provided by Shaanxi Power-Feed Technology Co., Ltd. The sows and piglets had free access to water and were immunised according to the company’s routine procedures.
Colostrum analysis
Within 2 h of the birth of the first piglet, eight sows per treatment were randomly selected for colostrum collection. Colostrum composition (milk fat, protein, lactose and casein, et al.) was determined via milk component analyzer (Foss FT 120), according to the manufacture’s instruction. Targeted fatty acid quantitation was defined with GC-MS by Novogene. Briefly, milk fatty acids were solvent-extracted using 1 % H2SO4 in methanol for 30 min at 80°C. The resulting methyl esters were extracted by N-hexane. FA methyl esters were separated and quantified using Thermo Trace and ISQ7000 GC-MS system equipped with a Thermo TG-FA methyl ester capillary column (50 m * 0·25 mm ID * 0·20 μm). The sample injection volume was 1 μl with a split ratio of 8:1. The temperature program was as follows: initial temperature 80°C for 1 min, increased by 20°C/min to final temperature 160°C and held for a further 1·5 min, increased by 3°C/min to final temperature 196°C and held for a further 8·5 min, increased by 20°C/min to final temperature 250°C and held for a further 3 min. The temperature of the interface was 250°C. Identification of fatty acid peaks was made by comparing their retention times to that of known FA methyl ester standards (Sigma-Aldrich). A 5-fold titration of mixture (containing fifty-one fatty acid methyl esters, 4000 μg/ml) was used to generate a standard curve. Individual fatty acid peaks were quantified as absolute values (mg/ml). Fatty acid composition in the serum of piglets was determined in a similar procedure as described above.
Blood analysis
On days 21 and 28, one piglet (close to the average body weight of litter) delivered from the sows (the same as those for foremilk sampling) was selected for blood sampling from the anterior vena cava. Serum biochemical indicators were analysed with a Beckman Coulter AU5800 biochemical analyzer (Beckman Coulter, Inc.). Serum growth factors, cortisol, immunoglobulins and intestinal endotoxins were determined by commercial kits (PoFAine IGF1, T3, T4, insulin, cortisol, IgA, IgG, IgM, zonulin, diamine oxidase and d-lactate ELISA kits) from Shanghai Hengyuan Biological.
Fecal microbial analysis
Fresh feces were collected directly by messaging the rectum of the corresponding piglets at day 21 of age. Stool samples were cooled on dry ice and stored at −80ºC until 16S rRNA sequencing by Biomarker Technologies. Microbial DNA was extracted with DNeasy PowerSoil kit as the protocol. The V3–V4 hyper variable regions within 16S rRNA were augmented with universal primers (338F-806R), and the amplicons were screened on Illumina Novaseq platform (Illumina) under Illumina’s standard procedure. The bioinformatic analysis was completed at the Biomarker biocloud platform. Briefly, paired-end reads were merged from the original DNA fragments by FLASH (version 1.2.7), which were further filtered by UCHIME. The clean reads were clustered into operational taxonomic units by USEARCH (version 10.0) on the basis of 97 % sequence similarity. All augmented tags were glossed and allied against SILVA database (version 132) through Ribosomal Database Project classifier (version 2.2, 80 % confidence threshold).
Diarrhoea index
Feces consistency (from days 22 to 28 post-weaning) was scored using a subjective score on a 3-point scale: 0, normal, well-formed and solid feces; 1, soft and formed feces; 2, sloppy, loose and semiliquid feces; 3, diarrhoea, watery and unformed feces. Piglets were considered to be diarrheic when the diarrhoea score was 2 or above(Reference Kelly, O’Brien and McCracken15). Diarrhoea rate (%) = Σ (the number of piglets/pen × days of diarrhoea)/(total number of trial piglets × 7 d). Diarrhoea index = the sum of diarrhoea scores/pen/(total number of trial piglets × 7 d).
Statistical analysis
Microbiota diversity was assessed based on the number of OUT and Shannon-index using the phyloseq package of R(Reference McMurdie and Holmes16), and differences in bacterial communities among diet groups were evaluated with permutational multivariate analysis of variance (PERMANOVA) using UniFrac distance matrices based on both unweighted and weighted UniFrac distances using QIIME 2 (2019.4)(Reference Caporaso, Kuczynski and Stombaugh17). All other data were analysed with one-way ANOVA procedure (SPSS 20.0 software for Windows, SPSS Inc.), statistical significance was declared at P < 0·05 and a trend towards significance was considered at 0·05 < P < 0·10. Post-hoc mean separation with a Tukey adjustment was performed when the ANOVA resulted in a P – valule < 0·05. Orthogonal polynomial contrasts were conducted to determine linear (L) and quadratic (Q) effects of supplemental fish oil. The interactions between the abundance of fecal microbiota and serum parameters were analysed by Spearman’s correlation.
Results
Maternal administration of fish oil altered fatty acid composition in colostrum and the blood of piglets
Dietary administration of fish oil from late gestation to lactation did not affect feed intake, body weight and backfat loss of the dams throughout the experiment (as listed in Supplementary Table S3), and the numbers of piglets born, born alive, healthy or weak or stillborn newborns, litter weight alive at parturition and average weight of piglets born alive were comparable among different groups (online Supplementary Table S4). Also, the addition of fish oil during late gestation and lactation did not significantly change the composition of colostrum regarding milk fat, protein, lactose or casein contents (Table 1). For fatty acid composition in colostrum, dietary supplementation of fish oil significantly heightened the concentrations of EPA, DHA and ω-3 PUFA in the colostrum with 3–5-fold, 5–8-fold and ∼2-fold increase, respectively, and led to significantly reduced ratio of ω-6/ω-3 PUFA as maternal intake of fish oil increased, while the content of ω-6 PUFA was not greatly altered (Table 2). Meanwhile, α-linolenic acid, another ω-3 PUFA, remained unaltered by maternal fish oil administration. In line with the changes in colostrum, plasma EPA and DHA in suckling piglets were significantly elevated by maternal exposure to fish oil in a dose-dependent way, and the ω-6/ω-3 PUFA ratio was dramatically decreased, especially in the lower (30 g/d) treatment group (Table 2).
Table 1. Effects of maternal fish oil on colostrum composition

Data were presented as mean ± sem, n 8.
Table 2. Effects of maternal fish oil on fatty acid composition in colostrum and blood of sucking piglets (μg/ml)

Values are medians unless otherwise indicated. ALA, α-linolenic acid.
a–bValues in rows with different superscript letters differ significantly (P < 0·05, n = 8).
Maternal administration of fish oil promoted the growth of piglets
To balance the carrying ability of each sow, newborns were cross-fostered within the same group, and thus the born litter size and weight were comparable among groups. Twenty-one days later, litter size at weaning was nearly the same, while the weaning weights of litters tended to be elevated by maternal intake of fish oil, reaching a significant level in the high dose group. The difference in litter weight gain was not significant owing to the higher variance within groups (Table 3).
Table 3. Effects of maternal administration of fish oil during late-pregnancy and lactation on growth of piglets

Values are medians unless otherwise indicated.
a–bValues in rows with different superscript letters differ significantly (P < 0·05, n = 15).
Serum IGF1 levels in suckling piglets fostered by sows treated with fish oil were significantly higher than those in the control group. Moreover, serum T3 was also significantly escalated by maternal supplementation of 30 g/d fish oil, whereas T4, insulin and cortisol remained unchanged (Table 4). The effects of maternal fish oil treatment on serum growth factors lasted for 1 week post-weaning, as significant increase was also observed in serum IGF-1, especially in the 60 g/d treatment group, and circulating T3 instead of T4 levels were significantly increased by the maternal intake of fish oil (Table 4). Notably, the responses of plasma IGF1 in offsprings to maternal fish oil were strictly dependent on dosages, more intake of fish oil, much higher IGF1 levels.
Table 4. Effects of maternal administration of fish oil during late-pregnancy and lactation on serum growth factors in piglets

Values are medians unless otherwise indicated.
a–bValues in rows with different superscript letters differ significantly (P < 0·05, n = 8).
Circulating cortisol, a well-known stress hormone, was not affected (P = 0·114) by the maternal addition of fish oil in nursing piglets but was significantly reduced by a third in weaned piglets (Fig. 1).

Fig. 1. Effects of maternal administration of fish oil during late pregnancy and lactation on serum concentration of cortisol in piglets. *Means significantly differ between groups (P < 0·05, n 8).
The concentration of IgG in colostrum tended to be increased by the maternal addition of fish oil, and a significant increase was observed in the high dose group (60 g/d, Table 5), while IgM (P = 0·882) and IgA (P = 0·150) in colostrum were not affected by maternal supplementation of fish oil. In the serum of suckling piglets (21 d of age), IgG and IgM were significantly elevated by maternal fish oil supplementation in a dose-dependent manner (Table 5), leaving IgA unchanged. However, 1 week post-wean, all of plasma IgG, IgM and IgA levels were significantly elevated by maternal supplementation with fish oil, and more addition of fish oil, more immunoglobins.
Table 5. Effects of maternal administration of fish oil during late-pregnancy and lactation on serum immunoglobulins content in colostrum and blood of piglets

Values are medians unless otherwise indicated.
a–bValues in rows with different superscript letters differ significantly (P < 0·05, n = 8).
Maternal supplementation of fish oil improved gut health of piglets
Zonulin and d-lactate in the blood of suckling piglets were significantly decreased by maternal treatment with fish oil, reaching a significant level with maternal supplementation of 60 g/d fish oil. Serum diamine oxidase (DAO) in the nursery piglets was also significantly reduced by maternal supplementation of fish oil, and a linear response of DAO to maternal intake of fish oil was observed. However, serum lipopolysaccharide (LPS) in suckling piglets was not largely affected by maternal administration of fish oil. Notably, 1 week post-wean (28 d of age), serum zonulin, DAO, D-lactate and LPS were significantly lower in fish oil offspring than those of the control; this was in accordance with the lower incidence of diarrhoea in piglets raised by fish oil sows compared with sows in the control group (Table 6). Neither serum C-reactive protein in nursing (P = 0·166) nor post-weaned piglets (P = 0·711) were affected by maternal fish oil supplementation.
Table 6. Effects of maternal administration of fish oil during late-pregnancy and lactation on serum endotoxins in piglets

Values are medians unless otherwise indicated. DAO, diamine oxidase; LPS, lipopolysaccharide; CRP, C-reactive protein.
a–bValues in rows with different superscript letters differ significantly (P < 0·05, n = 8).
As shown in Fig. 2(a), the Abundance-based Coverage Estimator (ACE), Chao1, Simpson and Shannon indices of fecal microbiota were similar among groups, and the PD_whole tree index was significantly increased by the maternal treatment with fish oil, suggesting that the α-diversity was largely increased by maternal supplementation with fish oil.

Fig. 2. Effects of maternal administration of fish oil during late-pregnancy and lactation on fecal microbiome in weaning piglets. *Means significantly differ between groups (P < 0·05, n = 8).
As shown in Fig. 2(b), at the phylum level, the enrichment of Chloroflexi and Acidobacteria was significantly increased by the low dose (30 g/d) but not the high dose (60 g/d) of fish oil. Furthermore, family Lactobacillaceae was significantly reduced by the low but induced by the high dose, and Acidaminococcaceae was significantly repressed by maternal supplementation of fish oil, regardless of the dosage. At the genus level, the changes in Lactobacillus were similar to that of Lactobacillaceae. The abundance of the genus uncultured_bacterium_f_Muribaculaceae was significantly increased by the high dose of maternal fish oil, while Phascolarctobacterium was significantly decreased by maternal fish oil under both high and low doses (Fig. 2(c)).
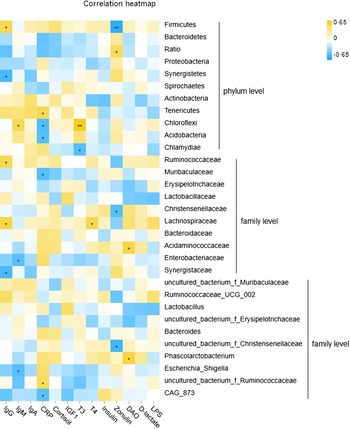
Pearson correlation analysis revealed a moderate and significant interaction between fecal microbiota and serum factors in near-weaning piglets (Fig. 3 and online Supplementary Table S5). At the phylum level, the abundance of Firmicutes was positively correlated with serum IgG and negatively correlated with serum zonulin, while the ratio of Bacteroidetes/Firmicutes was positively correlated with serum zonulin. Synergistetes was negatively associated with circulating IgG levels. Fecal Chloroflex was positively correlated with serum IgM and T3 concentrations. At the family level, Ruminococcaceae was positively correlated with serum IgG. Christensenellaceae was negatively correlated with circulating zonulin. Lachnospiraceae was positively correlated with both serum IgG and T4. Fecal Acidaminococcaceae was positively correlated with circulating DAO. Enterobacteriaceae was negatively associated with serum IgM, while Synergistaceae was negatively correlated with serum IgG. Notably, the genus uncultured_bacterium_f_Christensenellaceae was negatively correlated with serum zonulin. Phascolarctobacterium was positively associated with circulating DAO, and Escherichia-Shigella was negatively correlated with serum IgM.

Fig. 3. Correlation analysis between fecal microbiota and serum growth factors in suckling piglets. *P < 0·05; **P < 0·01.
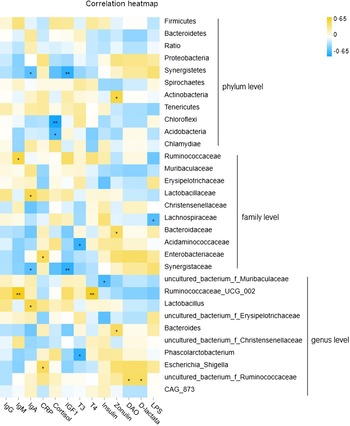
Moderate and significant interactions between fecal microbiota at weaning and post-weaning serum indicators were also observed (Fig. 4 and online Supplementary Table S6). At the phylum level, the abundances of Firmicutes, Bacteroidetes and the ratio of Bacteroidetes/Firmicutes were not significantly correlated with serum parameters in weaned piglets, although the phylum Synergistetes was negatively associated with serum IgA and IGF1 concentrations. Actinobacteria was positively correlated with circulating zonulin, and Chloroflexi and Acidobacteria were negatively associated with serum cortisol. At the family level, Ruminococcaceae was positively correlated with serum IgM, Lactobacillaceae was positively correlated with serum IgA; Lachnospiraceae was negatively associated with serum LPS; Bacteroidaceae was positively correlated with serum zonulin; Acidaminococcaceae was negatively correlated with serum T3 and positively with d-lactate; Enterobacteriaceae was positively correlated with serum C-reactive protein, and Synergistaceae was negatively correlated with IgA and IGF1.

Fig. 4. Correlation analysis between fecal microbiota and serum growth factors in weaned piglets. *P < 0·05; **P < 0·01.
At the genus level, uncultured_bacterium_f_Muribaculaceae was negatively correlated with circulating insulin; Ruminococcaceae_UCG-002 was strongly and positively correlated with IgG and T4 levels, and a positive interplay between Lactobacillus and serum IgA was observed. Moreover, Bacteroides was positively correlated with serum zonulin; Phascolarctobacterium was negatively associated with T3; Escherichia-Shigella was positively correlated with serum C-reactive protein, and uncultured_bacterium_f_Ruminococcaceae was positively interplayed with serum DAO.
Discussion
ω-3 PUFA, especially EPA and DHA, are essential fatty acids that can only be obtained from the diet, and insufficient intake of EPA or DHA can lead to a high risk of maternal under-nutrition and impaired fetal growth. Therefore an extra 200 mg/d DHA, as fatty acid from a sea fish once a week, is recommended for pregnant women(Reference von Schacky18). Maternal replenishment of marine oil has been documented to improve reproductive performance in sows(Reference Chen, Xu and Li14,Reference Petrone, Williams and Estienne19) and reduce inflammation in weaned piglets(Reference McAfee, Kattesh and Lindemann20), although the related mechanism remains unknown.
In the present study, maternal intake of fish oil during late gestation and lactation led to elevated contents of EPA and DHA in colostrum and piglets’ blood. Similarly, a double-blind, randomised, controlled trial in humans revealed that increased ω-3 PUFA in breast milk and infant DHA status was associated with supplementation during pregnancy(Reference Dunstan, Mitoulas and Dixon21). Furthermore, dose-dependent increases in plasma levels of EPA and DHA in offspring have been reported in shorthair cats(Reference Vuorinen, Bailey-Hall and Karagiannis22) and beagle dogs(Reference Dahms, Bailey-Hall and Sylvester23). Thus, maternal treatment of EPA and DHA could pass through to the next generation via colostrum and milk, elevating the intake of ω-3 PUFA in newborns.
Meanwhile, litter weight at weaning, especially in the high dose group, was tended to be increased upon exposure to fish oil in the prenatal and early postnatal life in this study, although the average birth weights were comparable, which was also observed in calves with a similar experiment design(Reference Jolazadeh, Mohammadabadi and Dehghan-Banadaky12). These data encouraged us to explore whether the endocrinal factors related to growth were altered by fish oil treatment, and results showed that exposure to fish oil in early life significantly escalated serum IGF1 and T3 in weaning and weaned piglets; furthermore, the increase of IGF1 and T3 by fish oil fits well in a linear model.
IGF1 is recognised as one of the predominant modulators during postnatal growth, whose expression was demonstrated to be associated with body height and loin muscle area in pigs(Reference Li, Wu and Ren24). A previous study showed that long-term (16 weeks) supplementation of fish oil significantly increased plasma IGF1 in 13- to 15-year-old boys(Reference Damsgaard, Mølgaard and Matthiessen25), and n-3 supplementation was documented to prevent the decrease of serum IGF1 induced by CVD in middle-aged men(Reference Gholamhosseini, Nematipour and Djazayery26) or a low-fat diet in postmenopausal women(Reference Young, Kurzer and Thomas27). In addition, short-term (11 d) of EPA administration was reported to reverse the circulating IGF1 reduced by arthritis in rats(Reference Castillero, López-Menduiña and Martín28). Considering that the liver is the major source of plasma IGF1 in postnatal piglets as a response to pituitary growth hormone plus(Reference Sawada, Arai and Jing29), the detailed mechanism concerning how ω-3 PUFA modulate hepatic IGF1 transcription, translation and secretion deserves further investigation.
In addition to IGF1, plasma T3 levels in the piglets were also dramatically increased by the maternal intake of fish oil in our study, in accordance with a recent survey in humans showing that prenatal exposure to fish oil was positively associated with the T3/T4 ratio in cord blood of newborns(Reference Wang, Cao and Gao30). However, an earlier study showed that maternal intake of fish oil during lactation exerted no significant effects on serum T3 or T4 in male offspring of rats(Reference Souza, Nunes and Paula31). Moreover, in a randomised double-blind controlled trial on obese subjects, treatment of DHA and EPA (ratio = 1:1·5) for 4 weeks significantly increased T4 but not T3 in fasting blood(Reference Taraghijou, Safaeiyan and Mobasseri32). These conflicting results led us to suppose that the thyroid status might be a secondary consequence of fish oil treatment and that this was influenced by other factors, for example, the cross-talk between thyroid-stimulating hormone and IGF1 receptor(Reference Krieger, Neumann and Gershengorn33).
Suckling-to-weaning transition always places piglets at high risk of severe stress thus impede growth. Notably, plasma concentrations of cortisol, stress hormone in pigs, were significantly reduced in weaned piglets nursed by sows fed with fish oil, compared with their counterparts in the control group, although the cortisol levels in weaning piglets were comparable, in accordance with a previous study where maternal replenishment of fish oil protected weaned piglets from acute inflammation induced by LPS challenge(Reference McAfee, Kattesh and Lindemann20). Thus, we suggest that the reduction of cortisol by fish oil might relieve harmful stress and facilitate the adaptation to weaning in piglets.
Fish oil has been long known to affect immunity. Unlike humans, IgG is the predominant immunoglobulin in porcine colostrum, which provides essential protection for the newborns, especially within the first 24 h postpartum when the gut is ‘open’(Reference Butler, Zhao and Sinkora34). Here, maternal supplementation of fish oil during late gestation and lactation tended to increase IgG in the colostrum, in line with a previous study where dietary administration of DHA plus EPA during late gestation and early lactation led to an increase in IgG(T) in mare’s milk(Reference Kouba, Burns and Webel35). Consistent with this, plasma IgG in suckling piglets was also elevated by exposure to fish oil in early life, a result observed in calves(Reference Jolazadeh, Mohammadabadi and Dehghan-Banadaky12) and foals(Reference Kouba, Burns and Webel35) with EPA and DHA supplementation in uterine and/or early life. Also, the level of circulating IgM was increased in suckling piglets along with IgG and IgA in weaned piglets born from sows supplemented with fish oil, which is an attracting result for us. The effective transfer of immunoglobins ceases when ‘gut closure’ occurs near weaning(Reference Butler, Zhao and Sinkora34), Thus, we hypothesised that the increased immunoglobins in weaned piglets, especially IgA, might be principally due to de novo synthesis.
IgA is the major immunoglobulin secreted by the parotid, nasal, bronchial and intestinal mucosa in piglets(Reference Butler, Zhao and Sinkora34). Fish oil has been demonstrated to protect weaned piglets from LPS challenge via enhancing intestinal barrier function(Reference Zhu, Liu and Chen36), and thus the increase of serum IgA in weaned piglets may be attributed to improve gut function. The gut barrier function of neonatal piglets is known to be relatively weak, gradually becoming strong as growing; exocrine secretion of three products from intestinal contents, zonulin, DAO and d-lactate, are commonly used as indicators of gut permeability(Reference Zou, Yang and Guo37). As expected, in our study, circulating zonulin, DAO and d-lactic acid in weaning and weaned piglets delivered from sows fed fish oil were significantly decreased compared with those of the control group. Moreover, plasma LPS, a typical endotoxin produced by pathogenic bacteria, was largely reduced by maternal fish oil supplementation, especially with the high-dose treatment in weaned piglets. Taken together, the results strongly suggested that maternal intake of fish oil was favourable to gut development and intestinal health of weaned piglets.
Gut health is tightly associated with the composition of the intestinal microflora; accumulating evidence has highlighted a substantial influence of dietary fish oil or ω-3 PUFA on the gut microbiome (see the latest review(Reference Fu, Wang and Gao8)). Moreover, the positive modulation of in utero and early life intake of ω-3 PUFA, particularly EPA and DHA, can extend to adolescence and adulthood(Reference Robertson, Seira Oriach and Murphy38). Thus, we moved on to explore the fecal microbiota structure in the weaning piglets.
Notably, the current data indicated that more or less exposure to maternal fish oil exerted different effects on the structure of the gut microbiota in the weaning piglets. For example, the family Acidaminococcaceae was dramatically reduced by maternal supplementation with fish oil and to a greater extent in the high-dosage treatment group, while the genus Phascolarctobacterium was reduced by the low dosage of fish oil. Interestingly, the abundance of Lactobacillus in the family Lactobacillaceae responded to fish oil in a binary linear pattern, being significantly reduced by the lower yet increased by the higher dosage of maternal fish oil. A newly published report suggests that dietary ω-3 PUFA could be partially metabolised by anaerobic Lactobacilli in the distal intestine of pigs, thus directly influencing the gut microbiota distribution(Reference Lauridsen1). As such, we assume that dietary ω-3 PUFA might not be entirely digested in the gut, as treatment with lower dosages of fish oil was not sufficient to promote or even repress the growth of Lactobacillus in present study.
The interplay between the gut microbiota and the host occurs in multiple and perplexing ways, and the functions and mechanic pathways of most microbiota species remain unconfirmed. A recent report showed that Akkermansia muciniphila, an emerging superstar against obesity and metabolic disorders, could secret an 84 kDa protein, P9 and the latter could induce systemic secretion of glucagon-like peptide-1, an important factor modulating energy metabolism(Reference Yoon, Cho and Yun39). Thus, we are curious whether gut microbiota impacts systemic immunity or serum growth-related factors in piglets.
Correlation analysis revealed an interaction between the gut microbiota and serum immunoglobulins. For example, Lachnospiraceae family was significantly and positively correlated with serum IgG in weaning piglets, which falls in line with an earlier study where Lactobacillus agents were shown to increase serum IgG in piglets(Reference Wang, Qiao and Wang40). The abundance of Escherichia coli-Shigella, the enrichment of which is relevant to human dysbacteriosis(Reference Lappan, Classon and Kumar41) and calf diarrhoea(Reference Ma, Villot and Renaud42), was negatively correlated with serum IgM in weaning piglets.
Although the composition of the gut microbiome undergoes a dramatic change during the suckling-to-weaning transition(Reference Beaumont, Cauquil and Bertide43), it was demonstrated that the gut microbiota at the time of weaning influenced the development of post-weaning diarrhoea(Reference Karasova, Crhanova and Babak44). Here, Lactobacillus in weaning piglets was positively correlated with serum IgA in weaned piglets, and the abundance of Ruminococcaceae_UCG_002, a butyrate producer(Reference Louis and Flint45) in weaning piglets, was positively correlated with serum IgM and T4 in weaned piglets. A significant and negative association between growth factor, IGF1 in weaned pigs, and the family Synergistaceae were also observed. It is noteworthy that the tendency of a negative correlation between Escherichia–Shigella and serum C-reactive protein in weaning piglets became statistically significant in weaned piglets. Although a causal relationship between the abundance of gut microbiota and serum indices cannot be confirmed until more rigorous experiments (fecal microbiota transplantation) are done, our data provides a hint that gut microbiota structure at weaning is tightly related to post-weaning growth and well-being of piglets, and strengthened the opinion that post-weaning growth performance and healthy status can be intervened through modulating the maternal diet by way of piglets’ gut microbiota(Reference McCormack, Curião and Wilkinson46).
It is worth mentioning that the establishment and maintenance of gut ecosystem in weaning piglets are determined by complex internal and external factors(Reference Guevarra, Lee and Lee3), especially the microbiota from their moms(Reference Chen, Ma and Jiang47). The intestine of neonates prior to birth is close to germ-free but rapidly shifts to a relatively complex microbial population(Reference Guevarra, Lee and Lee3), and the gut microbiota in piglets within 3 d after birth was predominantly affected by the vaginal microbiota of dams, then gradually affected by the sow fecal and slatted floor microbiota over time(Reference Chen, Ma and Jiang47). It is a shortage of the current experiment that we missed the vaginal swabs from the sows in labour, cautious stool and slatted floor samples during lactation, thus it is hard to trace the direct target microbiota in response to maternal fish oil, especially those possessing probiotic effects in piglets during suckling-to-weaning transition.
Conclusion
Our study demonstrated that maternal administration of fish oil during late pregnancy and lactation could be passed to the offspring via colostrum, thereby elevating serum levels of IGF1, T3 and immunoglobulins, reducing diarrhoea in weaned piglets, which appear to be associated with alteration of the gut microbial community.
Acknowledgements
This work was supported by National Key Research and Development Program of China (2021YFF1000602) and the Innovation Driven Program in Shaanxi Province (2021, #33).
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522003981