Phytic acid (PA; myo-inositol hexaphosphate) is a constituent of plant seeds, where it serves as a storage form of P(Reference Maenz, Bedford and Partridge1). Plant seeds form the bulk of pig and poultry feeds. PA-bound P in plant seeds (except in viscous grains like rye and wheat, which have high endogenous phytase activity) is, however, poorly digested by pigs and poultry because they do not produce sufficient amount of phytase enzyme that hydrolyses PA(Reference Bedford2).
Furthermore, each of the six phosphate groups on PA has two protons, which can dissociate to leave PA with twelve negative charges and hence the potential to bind positively charged molecules such as minerals and basic amino acids (AA)(Reference Maenz, Bedford and Partridge1). Of the twelve protons on PA, six can dissociate at acidic pH, three at neutral pH and the remaining three at basic pH, suggesting that PA has capacity to bind positively charged molecules in diet and endogenous gastrointestinal secretions such as digestive enzymes and mucin at all pH conditions found in the gut(Reference Maenz, Bedford and Partridge1).
PA has indeed been reported to limit the digestibility of minerals and AA in boilers(Reference Ravindran, Cabahug and Ravindran3–Reference Cowieson, Acamovic and Bedford5), which implies reduced efficiency of nutrient utilisation and increased environmental discharge of nutrients as a result of excessive excretion of unabsorbed nutrients due to PA(Reference Lenis and Jongbloed6). PA has also been shown to increase endogenous losses of minerals and AA in broilers(Reference Cowieson, Acamovic and Bedford7, Reference Cowieson and Ravindran8). Because the increased endogenous nutrient losses in the gut are associated with increased maintenance requirement for the lost nutrients and of the energy spent on their secretion(Reference Nyachoti, de Lange and McBride9), an increase in endogenous losses of nutrients due to PA imply that there are other adverse effects of PA on efficiency of nutrient utilisation in addition to reducing nutrient digestibility. However, there is a lack of information on the effect of PA on endogenous nutrient losses in pigs. Furthermore, the effect of PA on digestibility of nutrients, especially AA, has been variable. For instance, Bohlke et al. (Reference Bohlke, Thaler and Stein10) and Liao et al. (Reference Liao, Kies and Sauer11) reported a reduction in AA digestibility in pigs due to PA, whereas Johnston et al. (Reference Johnston, Williams and Southern12) and Woyengo et al. (Reference Woyengo, Sands and Guenter13) did not report improvement in AA digestibility due to phytase (which hydrolyses PA) despite increased PA hydrolysis as evidenced by improved P digestibility in their studies. The objective of the present study was to determine the effect of PA on the ileal mineral and AA digestibilities and endogenous flow of AA at the terminal ileum of weanling pigs.
Materials and methods
Animals
Seven Genesus (Yorkshire-Landrace female × Duroc male) barrows (mean initial body weight 9·50 (sd 0·42) kg) were obtained from Glenlea Swine Research Unit, University of Manitoba immediately after weaning, and group-housed in pens and monitored for consumption of a commercial starter diet to ensure that piglets were healthy and able to eat and hence ready for surgeries. After 3 d, piglets were transferred to metabolic crates to adapt to the crates for 2 d, and then surgically fitted with a simple T-cannula at the distal ileum as described by Nyachoti et al. (Reference Nyachoti, McNeilage-Van de Wiele and de Lange14). After surgery, piglets were returned to the metabolic crates and allowed a 7 d recovery period before the commencement of the experiment. During the recovery period, they were fed twice daily increasing amounts of the starter diet starting with 50 g of the feed after surgery and then increasing the amount offered by 50 g/d until feed consumption was at 2·6 times maintenance energy requirement(15) based on their body weight. Piglets had unlimited access to water throughout the study. The mean body weight at the start of the experiment was 10·9 (sd 0·48) kg. The use of animals in the present study was reviewed and approved by the University of Manitoba Animal Care Protocol Management and Review Committee (Protocol no. F05-001/1/2), and piglets were handled in accordance with the guidelines described by the Canadian Council on Animal Care(16).
Diets
During the experimental period, four casein–maize starch-based diets formulated to meet National Research Council(17) energy, essential AA, vitamin and mineral requirements for piglets (Table 1) were fed. The diets included a control diet either unsupplemented or supplemented with 5, 10 or 20 g/kg PA (as sodium phytate; Sigma-Aldrich Corporation, St Louis, MO, USA). Two sets of four diets were prepared: one set of diets contained chromic oxide (3 g/kg) as an indigestible marker and intact casein; whereas the other set contained titanium oxide (3 g/kg) as an indigestible marker and 50 % of the dietary casein was guanidinated to convert lysine to homoarginine for determination of endogenous AA flow at the terminal ileum by the homoarginine method(Reference Johnston, Williams and Southern12). Casein was guanidinated as described by Nyachoti et al. (Reference Nyachoti, McNeilage-Van de Wiele and de Lange14, Reference Nyachoti, de Lange and Schulze18).
Table 1 Composition of basal diet as fed basis*

* The basal diet was prepared in two sets; in one set, all the casein was intact, whereas in the other set, 50 % of casein in diet had been guanidinated.
† Chromic oxide was used as an indigestibility marker in un-guanidinated diet, whereas titanium oxide was used as an indigestibility marker in guanidinated diet.
‡ Vitamin/mineral premix supplied the following per kg of finished diet: retinol, 2479 μg; cholecalciferol, 25 μg; α-tocopherol, 13·4 mg; phylloquinone, 1·1 mg; riboflavin, 5 mg; nicotinamide, 36·8 mg; cyanocobalamin, 25 mg; pyridoxine, 4·4 mg; biotin, 200 mg; pteroyl(mono)glutamic acid, 1 mg; choline, 781 mg; copper, 6 mg; iodine, 0·28 mg; iron, 100 mg; manganese, 40; selenium, 0·30 mg; zinc, 100 mg.
§ Based on ingredient composition data by the National Research Council(17).
∥ Performed on un-guanidinated diet.
Experimental design and procedure
The experiment was conducted as a 4 × 4 Latin square design combined with a 4 × 3 Youden square design to give seven replicates per diet. Each period lasted for 7 d. Piglets were fed their respective un-guanidinated diets during the first 6 d and guanidinated diets during day 7 of each period. On days 5 and 6, ileal digesta was collected continuously from each pig from 08.00 to 20.00 hours daily and stored frozen at − 20°C for later determination of apparent ileal nutrient digestibility. On day 7, ileal digesta was collected continuously from 12.00 hours (when the chromic oxide (green colour) from un-guanidinated diets had disappeared from the digesta) to 10.00 hours the following day (before the appearance of chromic oxide from un-guanidinated diets fed on the same day at 08.00 hours) and similarly stored frozen for later determination of endogenous AA flow at the terminal ileum and true ileal digestibility (TID) of AA. During the experiment, pigs were fed the four experimental diets at 2·6 times maintenance energy requirement(16) based on their body weight at the beginning of each experimental period. The diets were offered in two equal portions at 08.00 and 15.30 hours on days 1–6 and in three equal portions at 08.00, 15.30 and 20.30 hours on day 7.
Sample preparation and chemical analyses
The ileal digesta collected from each pig in each period were pooled for days 5 and 6 (un-guanidinated diet) and for day 7 (guanidinated diet), homogenised in a blender (Waring Commercial, Torrington, CT, USA), sub-sampled and freeze-dried. The dried ileal digesta were finely ground in a grinder (CBG5 Smart Grind; Applica Consumer Products Inc., Shelton, CT, USA), and thoroughly mixed prior to analysis.
Diet and digesta DM content was determined according to Association of Official Analytical Chemists procedures (Procedure 4.1.06(19)), and N was determined using a N analyser (Model NS-2000; LECO Corporation, St. Joseph, MI, USA). Samples for Ca, P, K, Na and Mg analyses were ashed for 24 h and digested according to Association of Official Analytical Chemists(20) procedures (method 990.08) and read on a Varian Inductively Coupled Plasma Mass Spectrometer (Varian Inc., Palo Alto, CA, USA). Samples for AA analysis were prepared by acid hydrolysis according to Association of Official Analytical Chemists procedures (Procedure 4.1.11 alternative 3(19)). Samples for analysis of sulphur-containing AA (methionine and cysteine) were subjected to performic acid oxidation prior to acid hydrolysis. Tryptophan was not determined. Samples for Cr analysis were ashed and digested according to procedures described by William et al. (Reference William, David and Iismaa21) and read on a Varian Inductively Coupled Plasma Mass Spectrometer. Titanium was determined according to the method of Myers et al. (Reference Myers, Ludden and Nayigihugu22).
Calculations and statistical analysis
The extent of conversion of lysine to homoarginine was calculated as described by Nyachoti et al. (Reference Nyachoti, McNeilage-Van de Wiele and de Lange14). Apparent ileal DM, mineral and AA digestibilities, and TID of AA and endogenous AA flow at the terminal ileum were calculated as described by Nyachoti et al. (Reference Nyachoti, de Lange and Schulze18). Data obtained were subjected to ANOVA as a 4 × 4 Latin square design(Reference Cochran and Cox23) combined with a 4 × 3 Youden square design(Reference Cochran and Cox23) with 6 df for piglets, 3 df for periods, 3 df for diets and 15 df for the error term using Mixed procedure (SAS software release 9.1; SAS Institute, Cary, NC, USA). Linear and quadratic contrasts for unequally spaced levels(Reference Gill24) were performed to assess the effect of increasing dietary concentration of PA.
Results
The extent of conversion of lysine to homoarginine in guanidinated casein was 96·3 % (data not shown). Analysed chemical composition of the basal diet is shown in Table 1. The analysed crude protein and digestible lysine contents were similar to calculated values, whereas those for Ca and P contents and digestible methionine and threonine contents were higher than the calculated values.
The effects of dietary treatment on apparent ileal digestibility (AID) of DM, Ca, Na, K, Mg and P are presented in Table 2. PA linearly reduced (P < 0·05) the AID of DM. The AID of Na, K and P were linearly and quadratically reduced (P < 0·05) by increased dietary concentration of PA, whereas that of Ca and Mg was only linearly reduced (P < 0·05) by the dietary PA. The quadratic reduction in AID of P was such that the decrease in the digestibility was greater when the level of PA was increased from 0 to 10 g/kg than when it was increased from 10 to 20 g/kg. On the other hand, the quadratic reduction in AID of Na and K was such that the decrease in the digestibility of these minerals was lower when the concentration of PA was increased from 0 to 10 g/kg than when it was increased from 10 to 20 g/kg. The AID values for Na and K were negative when PA was supplemented at 20 g/kg, with the AID value for Na being the lowest.
Table 2 Effect of phytic acid level on apparent ileal DM and mineral digestibility coefficients in piglets fed casein–maize starch-based diet*

* For details of procedures, see Materials and methods.
† Linear effect (P < 0·05).
‡ Quadratic effect (P < 0·05).
Tables 3–5 show the AID, endogenous losses and TID of AA, respectively. Increasing dietary concentration of PA resulted in a quadratic response (P < 0·05) in AID of isoleucine, leucine, valine, glutamic acid, proline and serine such that the AID of these AA increased when the dietary concentration of PA was increased from 0 to 10 g/kg and then declined when the dietary PA level was further increased to 20 g/kg, though the differences between the AID values of the AA at dietary PA supplementation of 0 and 10 g/kg were marginal (on average by 1·8 percentage points). A similar trend was observed for TID of isoleucine, leucine, glutamic acid and proline when the dietary concentration of PA was increased. However, the AID and TID values of other AA, and the endogenous AA losses, were unaffected (P>0·05) by the dietary concentration of PA.
Table 3 Effect of phytic acid level on apparent ileal digestibility coefficients of nitrogen and amino acids in piglets fed casein–maize starch-based diet*
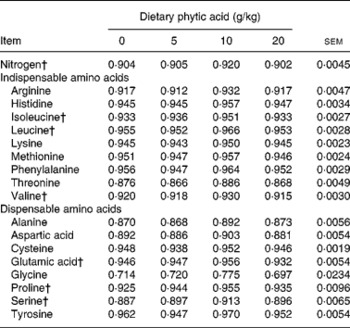
* For details of procedures, see Materials and methods.
† Quadratic effect (P < 0·05).
Table 4 Effect of phytic acid level on endogenous losses of amino acids (mg/kg DM) in piglets fed casein–maize starch-based diet*

* For details of procedures, see Materials and methods.
Table 5 Effect of phytic acid level on true ileal amino acid and nitrogen digestibility coefficients in piglets fed casein–maize starch-based diet*

* For details of procedures, see Materials and methods.
† Quadratic effect (P < 0·05).
Discussion
The analysed dietary Ca and P contents and digestible methionine and threonine contents were higher than the calculated values, which could probably be due to differences between the composition of the actual ingredients that were used in the present study and the values given by the National Research Council(17) for the same ingredients. The differences between the analysed dietary digestible methionine and threonine contents might also have been due to cumulative error during the analysis because the differences between the analysed and calculated values for crude protein and lysine were minimal.
PA has been shown to reduce AID of cationic minerals in pigs(Reference Bohlke, Thaler and Stein10) and broilers(Reference Ravindran, Morel and Partridge4), which is consistent with current observations. In the current study, however, PA supplementation at 20 g/kg resulted in negative AID values of Na and Mg, indicating increased endogenous flow of these minerals at the terminal ileum due to PA. Cowieson et al. (Reference Cowieson, Acamovic and Bedford7) also observed increased excretion of endogenous minerals in broilers due to PA. In both the present study and that of Cowieson et al. (Reference Cowieson, Acamovic and Bedford7), Na was the most affected mineral. Sodium deficiency in chickens has been reported to reduce the activity of intestinal Na-K-ATPase in the gut, which is involved in the absorption of glucose and other nutrients(Reference Gal-Garber, Mabjeesh and Sklan25). Thus, the increase in endogenous secretion of minerals such as Na due to PA may have significant nutritional and physiological implications as it could result in alteration of electrolyte balance and hence Na-K-ATPase activity and nutrient absorption in the small intestine. This is supported by results from a broiler study by Liu et al. (Reference Liu, Ru and Li26) which showed reduced activity of Na-K-ATPase in broilers due to ingestion of PA. However, in pigs unlike in poultry, the minerals that are endogenously secreted in the small intestine may be re-absorbed in the large intestine. Hence, the alteration in the electrolyte balance due to PA might be local (in the mucosa of the small intestine), but not systemic (in the whole body) as the deficiency of the minerals would be corrected by re-absorption in the large intestine. It will be interesting to see the effects of PA on Na retention in the body, and on the activity of Na-K-ATPase and nutrient absorption in the small intestine.
The mechanisms by which PA increases the endogenous secretion of minerals in the gastrointestinal tract have not yet been established. However, two mechanisms are proposed. First, PA may bind to enzyme co-factors in the gut, resulting in increased mineral secretion through negative feedback mechanisms. Second, amino groups found on side-chains of basic AA and at amino terminal ends of proteins possess a net positive charge at pH below the isoelectric point(Reference Hídvégi and Lásztity27). Hence PA may interact with dietary protein, and endogenous protein (pepsin, and pepsinogen and its activating peptide) in the stomach, where the pH is acidic(Reference Cowieson, Acamovic and Bedford7), thereby reducing the activity of pepsin and hence increasing the secretion of the enzyme and HCl via negative feedback mechanisms(Reference Cowieson, Acamovic and Bedford5). The resulting acidic digesta may then need to be neutralised in the small intestine by mineral-bicarbonates secreted by the intestine(Reference Allen, Flemstrom and Garner28) and pancreas(Reference Zebrowska, Low and Zebrowska29) to protect small intestinal mucosa from acid digestion and to optimise pancreatic and intestinal digestive enzymes, resulting in increased endogenous secretion and hence losses of the minerals. Because pancreatic juice is richer in Na than other minerals(Reference Zebrowska, Low and Zebrowska29) and it is Na that was most affected by PA, it appears that PA increases the endogenous losses of minerals (especially Na) mainly by increasing the endogenous secretion of mineral-bicarbonates in the small intestine to neutralise the acidic digesta exiting the stomach. This hypothesis, however, needs to be proven.
In the current study, the reduction in AID of monovalent cations (Na and K) due to increased dietary concentration of PA was quadratic such that the reduction in AID of these cations was lower when the dietary PA level was increased from 0 to 10 g/kg, than when it was increased from 10 to 20 g/kg. On the other hand, the reduction in AID of divalent cations (Ca and Mg) due to dietary PA was linear such that the magnitude by which dietary PA reduced the AID of these cations did not change when the level of PA was increased from 0 to 10 g/kg or from 10 to 20 g/kg. PA is known to form weaker bonds with monovalent cations than with divalent cations(Reference Erdman30). This implies that the reduction in AID of cations due to their binding to dietary PA could be more for divalent than monovalent cations. Therefore, the different responses in the AID of monovalent cations compared with that of their divalent counterparts (due to increasing level of dietary PA) could probably be due to differences in mechanisms by which PA reduces the AID of the two categories of cations (monovalent versus divalent).
PA supplementation reduced the AID of P in the current study. This is interesting because P, unlike other minerals whose AID was measured in the current study, is negatively charged. The reduced AID of P due to PA could have been due to binding of PA to both endogenous and dietary non-PA P via divalent cations to form PA–cation–P complexes. Because the true digestibility of P in pigs is lower for diets with higher PA concentration than for those with lower concentration of the same(Reference Dilger and Adeola31), the lower AID of P due to PA could also have been as a result of higher content of PA-bound P in PA-supplemented diets. It is, however, not clear why the decrease in AID of P, in contrast to that of other minerals measured in the present study, was greater when the level of PA was increased from 0 to 10 g/kg than when it was increased from 10 to 20 g/kg.
The rate of conversion of lysine into homoarginine in casein (96·3 %) was within the range of values reported by Nyachoti et al. (Reference Nyachoti, McNeilage-Van de Wiele and de Lange14) (89·2 %) and Schmitz et al. (Reference Schmitz, Hagemeister and Erbersdobler32) (99·6 %). The AID, endogenous losses and TID of AA observed in the current study were generally similar to what Nyachoti et al. (Reference Nyachoti, de Lange and Schulze18) reported in growing pigs fed casein–maize starch with casein content (20 %) that is similar to what was contained in the basal diet used in the current study.
In addition to reducing mineral digestibility, PA is expected to bind to AA in the diet and proteolytic enzymes(Reference Cowieson, Acamovic and Bedford7), resulting in reduced true AA digestibility. Also by interacting with AA in both diet and proteolytic enzymes in the stomach, PA is expected to increase enzyme and HCl secretion as previously discussed. Also, PA could bind to enzymes secreted by pancreas and small intestinal wall, resulting in their compensatory secretion. Because one of the physiological functions of mucin is to protect the gut wall from degradation by acid and proteolytic enzymes(Reference Montagne, Piel and Lalles33), the increased proteolytic enzymes and HCl secretion is expected to result in increased mucin secretion to protect the gut wall from the enzyme and acid digestion, resulting in increased endogenous losses of AA and N contained in the digestive enzyme and mucin. Thus, the presence of PA in pig diets is expected to result in reduced TID and increased endogenous losses of AA, and hence decreased AID of the same. In the current study, however, PA supplementation did not decrease the AID and TID of AA and promote the endogenous losses of the same. This observation contradicts results of Bohlke et al. (Reference Bohlke, Thaler and Stein10) and Liao et al. (Reference Liao, Kies and Sauer11), who reported decreased AID of AA due to PA. Results of the current study, however, are similar to those of studies that did not report reduced AA digestibility in pigs due to phytase (which hydrolyses PA) despite increased PA hydrolysis as evidenced by improved P digestibility(Reference Johnston, Williams and Southern12, Reference Woyengo, Sands and Guenter13). It is not clear why PA has variable effects on AA digestibility in pigs.
Naturally, PA occurs as a mixed salt of cations, mainly K and Mg, and to a lesser extent Ca, Fe and Zn in spherical inclusions called globoids within protein bodies(Reference Ockenden, Dorsch and Reid34–Reference Prattley and Stanely37). In most feed ingredients, PA is concentrated within cells that are rich in fibre(Reference Ockenden, Dorsch and Reid34, Reference Joyce, Deneau and Peterson35). Thus, in order to determine the actual effect of PA on nutrient utilisation, PA-free diet should be fed with and without PA in a fibre-free form because fibre also affects the digestibility and endogenous losses of nutrients. It was, however, difficult to get PA in a form that is naturally found in feed ingredients (mixed salt of cations) in the market. Most of the PA products that were available in the market at time when the study was conducted are salts of one cation and hence the reason why sodium phytate was used in the current study. Nonetheless, in pigs, the mode of action of intrinsic PA is expected to be the same as that used in the present study due to the following two reasons. First, PA and the cationic minerals disassociate at acidic pH found in the stomach because they are both soluble at this pH(Reference Maenz, Bedford and Partridge1). Therefore, the form in which PA exerts its effects in the stomach is independent of the original form in which it is supplied in the diet. Second, at small intestinal pH, PA reacts with free cationic minerals to form phytate(Reference Maenz, Bedford and Partridge1). The major cationic mineral in practical diets is Ca. Therefore, PA is likely to complex more Ca than other cations regardless of its original form in the diet. It should, however, be noted that the pH in the crop of poultry is not acidic enough to cause disassociation between PA and cationic minerals, and therefore, PA may exert its effects in the crop in a form that is similar to the form that it is supplied in the diet, resulting in an overall influence of dietary form of PA on response to its ingestion.
In conclusion, the results suggest that dietary PA has limited effect on the digestibility and endogenous losses of AA, but can reduce apparent ileal mineral digestibility in piglets partly due to their increased endogenous secretion at the terminal ileum as evidenced by negative AID values for some minerals. The increase in endogenous flow of minerals such as Na may be particularly important as an increased presence of these minerals in the gut will effectively alter electrolyte balance. The altered electrolyte balance may result in reduced activity of Na-K-ATPase and hence the capacity of the enterocytes to transport glucose and other nutrients. The present data are thus suggestive of important physiological responses in piglets to the ingestion of PA.
Acknowledgements
The research was conducted at the University of Manitoba. The authors thank R. Stuski for assistance with animal care and Dr G. Crow for statistical consulting. This work was supported by Manitoba Pork Council through its strategic research funding to the Department of Animal Science, University of Manitoba. None of the authors had any conflict of interest. T. A. W. designed and conducted the study, analysed data and prepared the first draft of the manuscript. A. J. C. and O. A. critically reviewed the experimental protocol and the manuscript, whereas C. M. N. provided overall direction for the study and manuscript preparation.







