Gastric ulcers (GU) are a prevalent digestive disorder that affects individuals globally(Reference Ford, Gurusamy and Delaney1). Psychological stress, Helicobacter pylori infection, alcohol consumption, tobacco smoking and nonsteroidal anti-inflammatory drugs (NSAID) are all contributing factors to GU formation. NSAID alone contribute to 25 % of incidence(Reference Adhikary, Yadav and Roy2). Indomethacin (IND) mainly treats inflammatory diseases such as rheumatoid arthritis, osteoarthritis and tendonitis. IND is also widely used for experimental induction of GU due to its higher ulcerogenic potency in comparison with other NSAID(Reference Lucas3,Reference Suleyman, Albayrak and Bilici4) . Several mechanisms are proposed to explain the harmful effects of IND on the stomach. These include inhibition of the cyclooxygenase enzyme, reduced synthesis of mucus and bicarbonate, impaired release of the gastroprotective prostaglandin E2, enhancement of acid production and oxidative stress(Reference Takeuchi5). Recent studies have found a correlation between autophagy and apoptosis with mucosal erosions and ulcerations caused by IND(Reference Gebril, Ito and Shibata6).
Certain natural products possess antioxidant and anti-inflammatory properties that may protect against gastrointestinal inflammation(Reference Hassan, Kamal and Nassar7–Reference Repetto and Llesuy9). Gamma-linolenic acid (GLA), also known as cis 6, cis 9, cis 12-octadecatrienoic acid, is a type of fatty acid belonging to the n-6 family. Although the body can produce it from the essential fatty acid linoleic acid, it is conditionally essential. Only trace amounts of GLA can be found in many plants, and it is not typically present in most commercial vegetable seed oils. However, certain species in families such as Saxifragaceae, Aceraceae, Boraginaceae, Cannabinaceae, Onagraceae, Liliaceae, Ranunculaceae and Scrophulariaceae contain GLA. Few plant sources have been used for commercial GLA production, mainly in health food, pharmaceutical, pet food and cosmetic industries. These sources include oils from borage, evening primrose, black currant and, more recently, hemp(Reference Kapoor and Nair10). The GLA treatment effectively inhibits the growth of gastric cancer cells in hypoxia. It achieves this by reducing cell viability and colony formation while increasing apoptosis. Additionally, GLA treatment regulates the expression of epithelial and stromal marker proteins and inhibits both cell migration and invasion. Furthermore, it reduces the expression of b-catenin in the Wnt/b-catenin pathway(Reference Wang, Shi and Gong11). A study compared GLA and linoleic acid effects on aspirin-induced gastric bleeding in rats. The mice that were fed with a GLA-enriched diet did not experience bleeding, whereas the mice that were fed with a linoleic acid-enriched diet did. This study suggests that GLA may protect the gastric mucosa from aspirin-induced damage by bypassing the decrease in delta-6 saturation and providing precursors for the synthesis of arachidonic acid and prostaglandins(Reference Huang, Drummond and Horrobin12).
Although GLA has been shown to have beneficial effects on the gastrointestinal tract, there is still incomplete information regarding its protective effect on GU. Our objective is to investigate the impact of GLA on GU caused by it and to identify its potential mechanisms.
Methods
Animals
Male Wistar rats (250–260 g) were obtained and allowed 1 week for adaptation. The rats were kept under a 12-h light/dark cycle with a temperature of 22 ± 2°C. They were fed standard chow pellets and had unlimited access to water. The study was conducted following the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and ARRIVE guidelines for reporting in vivo experiments(Reference Percie du Sert, Hurst and Ahluwalia13). The experimental protocol was approved by the Research Ethics Committee of the Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran (Protocol No. EE/1401.2.24226203/scu.ac.ir).
Study design
Thirty rats were randomly divided into five groups, with each group comprising six rats. The control and IND groups were administered sunflower oil through oral gavage for 14 consecutive days. The rats in GLA 100 and 150 mg/kg groups were orally administered GLA (100 or 150 mg/kg) (12) dissolved in sunflower oil (15 % saturated, 85 % unsaturated fatty acid and consisting of 14–43 % oleic and 44–75 % linoleic acids(Reference Flagella, Rotunno and Tarantino14)) for 14 successive days. The rats in the omeprazole 20 mg/kg group were orally administered omeprazole 20(Reference Rahimi, Shirvani and Sanaie15) for 14 successive days. On day 14 of the experiment, all rats except those in the control group were given a single oral dose of IND (50 mg/kg)(Reference Shaik and Eid16). The rats were not allowed to eat but had access to water for 24 h before receiving the IND. Rats were anesthetised with thiopental Na (50 mg/kg, i.p.) and euthanised by decapitation 4 h after IND administration. The stomachs from each group were then isolated (Fig. 1). GLA was obtained from evening primrose (Oenothera biennis L.) oil manufactured by Barij Essence Pharmaceutical Company in Iran.

Fig. 1. Flow chart of the included cases and controls.
Assessment of gastric pH
The gastric pylorus was tied off and a section of the stomach was removed through the greater curvature. The stomach contents were collected immediately and transferred to a centrifuge tube. The tube was then centrifuged for 5 min at 2500 × g to remove any debris before measuring the amount of supernatants. The pH of the gastric juice was measured using a digital pH meter.
Assessment of ulcer index and inhibition index
After removing the stomachs, they were sliced along the greater curvature and rinsed with 5 mL of normal saline. A blinded evaluation of gross lesions was performed. The mean number of ulcers per stomach for each rat was calculated using an ulcer scoring system for each group. 0 means no ulcer, 1 means pinpoint ulcers and superficial mucosal changes, 2 means ulcers smaller than 1 mm, 3 means ulcers between 1 mm and 2 mm and 4 means ulcers larger than 2 mm or a perforated ulcer.
((UIcontrol - UItreated)/UIcontrol) × 100, where UI represents ulcer index.
Histopathological examination
Stomachs were fixed in 10 % formaldehyde solution then dehydrated, embed them in paraffin and, finally, cut them into 4 μm sections using a microtome. These sections were then stained with haematoxylin and eosin (H&E) for histopathological examination.
Biochemical measurements
ELISA methods were utilised to determine various biochemical and antioxidant parameters in the gastric tissues. The parameters included prostaglandin E2 (PGE2), cyclooxygenase 1 (COX-1), TNFα, IL-6, intercellular adhesion molecule-1 (ICAM-1), malondialdehyde (MDA), as well as antioxidant parameters such as superoxide dismutase (SOD), glutathione (GSH) and catalase (CAT). All samples were tested for total protein content by the Bradford method. The test kits used for PGE2, COX-1 and ICAM-1 were purchased from MyBioSource in the USA, while those for TNF-α, IL-6, MDA, SOD, GSH and CAT were obtained from Kiazist in Hamedan, Iran.
Statistical analysis
We used SPSS software version 26 to analyse the data. First, we conducted a normalisation test on the data to ensure that it was normally distributed and had homogeneity of variances, using the Kolmogorov–Smirnov test on SPSS. As the data passed the normality test, we then performed a one-way ANOVA to compare the groups. We conducted post hoc analyses utilising Tukey tests. Ulcer index data were analysed by the Kruskal–Wallis test. We considered a significance level of P < 0·05.
Results
Effect of Gamma-linolenic acid pre-treatment on macroscopic inspection of gastric mucosa
According to the results of this study, it was found that the groups treated with IND, GLA 100 mg/kg and GLA 150 mg/kg had a significantly higher average ulcer score compared with the control group (P < 0·001, P < 0·05 and P < 0·05, respectively). However, there was no significant difference in the mean ulcer score between the omeprazole group and the control group. Conversely, the groups treated with GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg omeprazole had a significantly lower average ulcer score compared with the IND group (P < 0·01). This can be seen in Figs. 2(a) and (d).
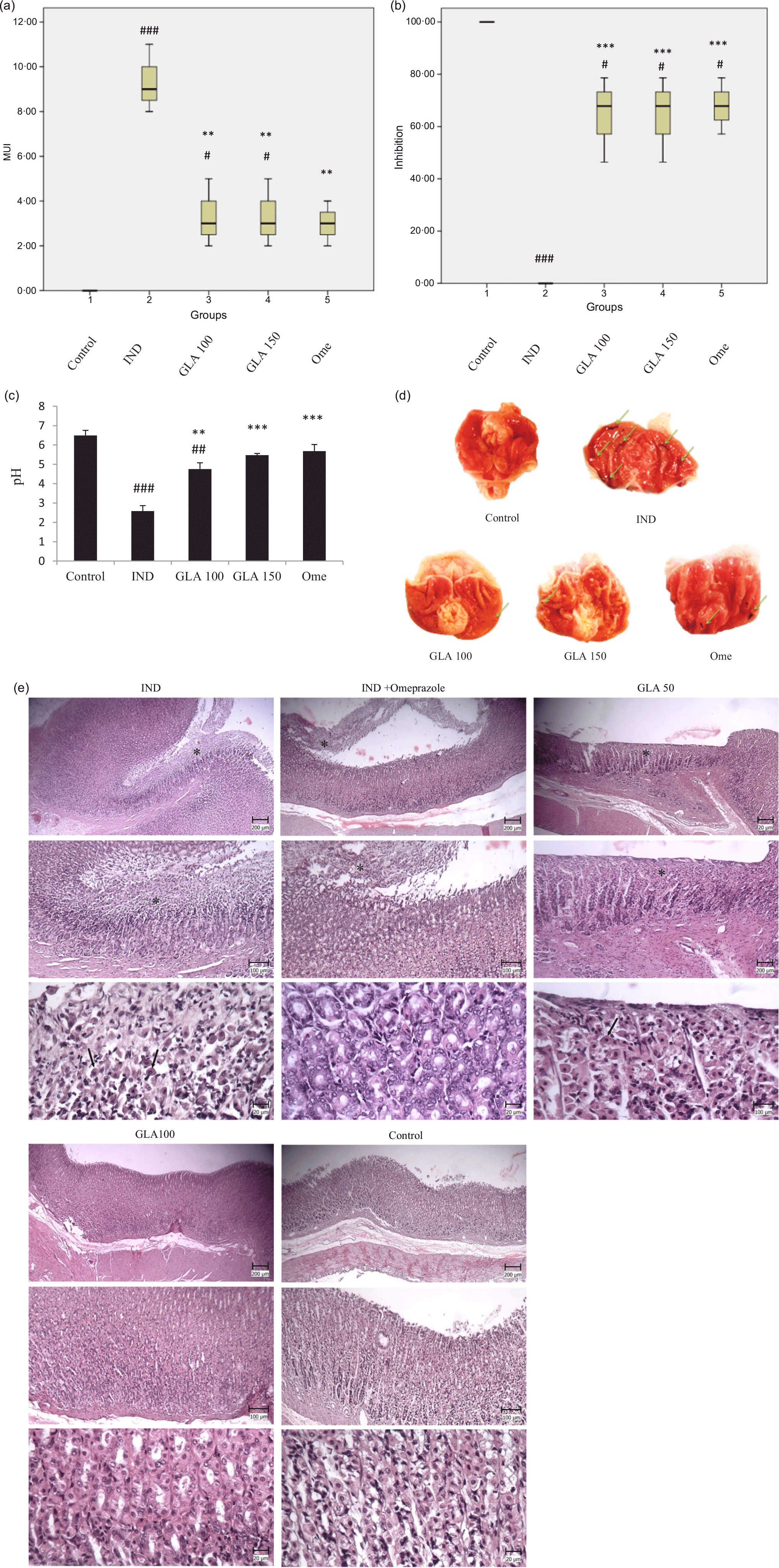
Fig. 2. Restricted cubic splines nested in logistic regression analyses for associations of NAFLD risk with serum levels of (a) TMAO, (b) choline, (c) trimethylamine, (d) L-carnitine, (e) betaine and (f) DMG.
The study found that the control group had a significantly higher percentage of ulcer inhibition than the groups given IND, GLA 100 mg/kg, GLA 150 mg/kg and omeprazole 20 mg/kg (P < 0·001, P < 0·05, P < 0·05 and P < 0·05, respectively). On the other hand, the groups given GLA 100 mg/kg, GLA 150 mg/kg and omeprazole 20 mg/kg had a significantly higher percentage of ulcer inhibition compared with the IND group (P < 0·001) (Figs. 2(b) and (d)).
Effect of Gamma-linolenic acid pre-treatment on gastric pH
The study revealed that groups treated with IND and GLA 100 mg/kg had a lower pH level of gastric juice compared with the control group (P < 0·001 and P < 0·01, respectively). However, there was no significant difference in the pH level of gastric juice between the GLA 150 mg/kg and omeprazole 20 mg/kg groups and the control group. On the other hand, the groups treated with GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg omeprazole had a higher gastric juice pH level than the IND group (P < 0·01, P < 0·01 and P < 0·001, respectively). Figure 2(c) depicts this information.
Effect of Gamma-linolenic acid on histopathological assessment
Microscopic examination of the stomach sections in the control sunflower group showed the absence of extensive changes in the mucosal surface. In most cases, cylindrical mucous cells with clear cytoplasm were visible. In some cases, hyperaemia was also seen in the parenic capillaries. In the microscopic examination of stomach sections in the group receiving IND, multiple, wide and deep wounds were observed on the mucosal surface. In these foci, gastric mucous cells, main and borderline, were necrotic, which were characterised by more colourful cytoplasm and compact and dark-coloured nuclei. Also, in some parts, the mucous cells were removed. This tearing continued to the deep parts of the stomach pits as well (Fig. 2(e)).
In the GLA50 treatment groups, wounds were still seen on the surface of the mucosa. In these foci, similar to the previous group, necrosis of mucous cells, main and border cells were seen. In the GLA100 treatment group, a large part of the mucosa was healthy the mucosal tissue had complete integrity, and only small foci of mucosal cell necrosis were seen. In the group receiving indomethacin and omeprazole, as in the previous group, a wide range of gastric mucosa was healthy, and small foci of surface necrosis were observed (Fig. 2(e)).
Effect of Gamma-linolenic acid pre-treatment on the levels of gastric prostaglandin E2, cyclooxygenase 1, TNF-a, IL-6 and intercellular adhesion molecule-1 content
The PGE2 levels were significantly higher in the groups that received GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole compared with the IND group. The P values for this difference were also less than 0·001. Conversely, the groups that received IND, GLA 100 mg/kg and GLA 150 mg/kg showed significantly lower levels of PGE2 than the control group (P < 0·001, P < 0·05 and P < 0·05, respectively). These findings are presented in Fig. 3(a).

Fig. 3. Receiver operating characteristic curves of traditional risk factors (blue) plus TMAO, choline and its related metabolites (red) for NAFLD.
The groups given GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole had significantly higher COX-1 levels compared with the group given IND (P < 0·05, P < 0·01 and P < 0·05, respectively). However, the groups given IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole showed significantly lower levels of COX-1 than the control group (P < 0·001) (Fig. 3(b)).
The TNF-α levels in the GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg omeprazole groups are significantly decreased compared with the IND group (P < 0·001). Additionally, the levels of TNF-α are significantly higher in the groups given IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole, compared with the control group (P < 0·001), as shown in Fig. 3(c).
The IL-6 levels in the GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg omeprazole groups are significantly decreased compared with the IND group (P < 0·001). Furthermore, the groups given IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole have significantly higher levels of IL-6 compared with the control group (P < 0·001, P < 0·001, P < 0·01 and P < 0·001, respectively), as shown in Fig. 3(d).
The ICAM-1 levels in the groups that received GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole were significantly decreased compared with the IND group (P < 0·05, P < 0·01 and P < 0·01, respectively). The group that received IND showed significantly higher levels of ICAM-1 compared with the control group (P < 0·001). However, there was no significant difference in the levels of ICAM-1 between the groups that received GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole and the control group (Fig. 3(e)).
Effect of Gamma-linolenic acid pre-treatment on the oxidative stress markers
The study found that IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg omeprazole increased MDA levels compared with the control group (P < 0·001, P < 0·05, P < 0·01 and P < 0·01, respectively). However, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg omeprazole reduced MDA levels compared with IND (P < 0·01) (Fig. 4(a)).

Fig. 4. Indirect effects of trimethylamine-N-oxide on the association of NAFLD risk with serum levels of (a) L-carnitine, (b) betaine and (c) DMG. *P < 0·001.
The results showed that the groups treated with IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole experienced a significant decrease in SOD levels compared with the control group (P < 0·001). However, SOD levels in the groups treated with GLA 100 mg/kg, GLA 150 mg/kg and omeprazole 20 mg/kg were found to be significantly higher than the IND group (P < 0·001) (Fig. 4(b)).
The groups that received IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole demonstrated a significant decline in GSH levels when compared with the control group (P < 0·001). However, the groups that were treated with GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole had significantly higher GSH levels than the IND group (P < 0·05, P < 0·01 and P < 0·01, respectively), as depicted in Fig. 4(c).
According to the findings, the groups that received IND, GLA 100 mg/kg, GLA 150 mg/kg and 20 mg/kg of omeprazole had notably lower CAT levels compared with the control group (P < 0·001). However, the CAT levels in the groups that were administered GLA 100 mg/kg, GLA 150 mg/kg and omeprazole 20 mg/kg were significantly higher than the IND group (P < 0·001) (as shown in Fig. 4(d)).
Discussion
PUFA such as α-linolenic acid (18:3n-3) and γ-linolenic acid (GLA, 18:3n-6) are beneficial for human health(Reference González-Fernández, Ortea and Guil-Guerrero17). In some cases, GLA had a better effect, while in others, GLA and α-linolenic acid had similar effects(Reference González-Fernández, Ortea and Guil-Guerrero17,Reference Yadav, Tiwari and Singh18) . In a related study, researchers suggest that GLA treatment may protect the gastric mucosa from aspirin-induced damage by providing a precursor for the synthesis of arachidonic acid and prostaglandins, bypassing the decrease in delta-6 desaturation(Reference Huang, Drummond and Horrobin12). Supplementing with GLA, EPA and DHA has been shown to effectively increase Dihomo-γ-linolenic acid (DGLA), arachidonic acid, EPA and DHA levels in equine plasma and erythrocytes, which may prevent or resolve severe stomach ulcers(Reference Pagan, Hauss and Pagan19).
After being produced in the body, GLA rapidly elongates into DGLA which can be converted into either anti-inflammatory (1-series prostaglandins) or pro-inflammatory products (2-series prostaglandins and 4-series leukotrienes)(Reference Mustonen and Nieminen20,Reference Fan and Chapkin21) . When there is limited activity of Δ5-desaturase, DGLA is changed to arachidonic acid. However, when dietary GLA supplementation occurs, DGLA can accumulate instead of ARA in many cell types. DGLA has two pathways of metabolism – the cyclooxygenase (COX-1 and COX-2) pathway and the 15-lipoxygenase pathway. In the COX pathway, DGLA turns into 1-series prostaglandins, especially PGE1. While in the lipoxygenase pathway, DGLA gets converted to 15-(S)-hydroxy-8,11,13-eicosatrienoic acid (15-HETrE). Both metabolites have been shown to have several benefits, such as reducing inflammation, promoting vasodilation, preventing smooth muscle cell proliferation, decreasing blood pressure and having anti-cancer effects(Reference Mustonen and Nieminen20). In our study, pretreatment with GLA increased both COX-1 and PGE-2 in the IND-induced gastric ulcer model, while IND decreased them. According to Henry and Robertson (1993), among NSAID, IND is the most ulcerogenic to humans(Reference Henry and Robertson22). It has been observed to induce a range of pro-inflammatory mediators and inhibit the gastroprotective COX-1 and angiogenesis in gastric tissue(Reference Yadav, Adhikary and Chand23). Additionally, NSAID contribute to gastric mucosal damage by causing oxidative stress and generating reactive oxygen species (ROS)(Reference Utsumi, Yasukawa and Soeda24). Blocking COX enzymes also inhibits the protective effects of PGE2, which include increased mucus and bicarbonate secretion, as well as an increase in gastric blood flow(Reference Asako, Kubes and Wallace25).
IND can cause oxidative stress that leads to inflammation and the production of pro-inflammatory mediators such as IL-6 and TNF-α (Reference Rahman26). The oxidative stress brought about by IND may also cause mitochondrial respiration uncoupling, resulting in the production of pro-inflammatory cytokines like TNF-α and IL-6. These cytokines can promote the expression of adhesion molecules such as ICAM-1, which play a significant role in the development and advancement of injury and inflammation in the gastric tissue(Reference Bindu, Mazumder and Dey27). ICAM-1 is responsible for the adhesion of leukocytes and endothelial cells after an injury(Reference Bella, Kolatkar and Marlor28). In this study, pre-treatment with GLA significantly reduced the levels of IL-6, TNF-α and ICAM-1 in rats exposed to IND, effectively mitigating inflammation. These findings align with previous research which demonstrated that GLA can decrease the production of inflammatory cytokines(Reference Chang, Sun and Lii29).
The present study found that IND can induce oxidative stress, as shown by decreased GSH content and SOD and CAT activity in gastric tissue and a significant increase in MDA concentration. These results are consistent with previous studies, which suggest that IND is involved in the production of ROS and associated gastric mucosal apoptosis(Reference Dursun, Bilici and Albayrak30–Reference Chung, Bae and Lee33). It’s interesting to note that GU occur due to a high concentration of ROS, including hydroxyl radicals, hydrogen peroxide and superoxide anions. These ROS cause oxidative stress in the gastric tissue, which leads to gastric bleeding and ulcer development, according to Repetto and Llesuy 2002(Reference Repetto and Llesuy9). However, intracellular antioxidant enzymes like catalase can counteract the harmful effects of ROS. Also, GSH can prevent tissue damage by neutralising ROS. Oxidative stress can increase lipid peroxidation and produce MDA, which is commonly used as a marker for lipid peroxidation(Reference Dursun, Bilici and Albayrak30,Reference Al Batran, Al-Bayaty and Jamil Al-Obaidi34) . In our study, GLA increased the levels of SOD, GSH and catalase and decreased MDA in GU induced by IND compared with the model group.
Conclusion
The administration of GLA at doses of 100 and 150 mg/kg was found to decrease oxidative stress markers. Additionally, the gastroprotective effect of GLA at these doses could be attributed to the reduction of TNF-1, IL-6 and ICAM levels, as well as the increase of PGE2 and COX1 levels. It seems that GLA strengthens the defense barrier against IND damage by boosting antioxidant levels, reducing oxidant reduction and decreasing inflammatory factors.
Acknowledgement
We are grateful to the Research Council of Shahid Chamran University of Ahvaz (GN: SCU.VB1402.50857).
This research did not receive any grant from funding agencies.
K. R. conceptualised and designed the study; K. R., M. E. G., A. R., M. H. and Y. S. A. analysed and interpreted the data; K. R. supervised the project and all authors have approved the final version of the manuscript.
There are no conflicts of interest.







