A 49-year-old woman underwent epilepsy surgery evaluation for the treatment of refractory epilepsy. Her seizures had begun at the age of 32, within 1 year of being diagnosed with and treated for cervical cancer. Brain MRI performed as part of epilepsy surgery evaluation was unremarkable, while continuous EEG showed predominantly right temporal lobe epileptiform activity. Brain PET showed subtle right hemispheric hypometabolism involving the right temporal lobe. Given her medically refractory seizures and her history of malignancy in close temporal relationship to seizure onset, her epileptologist submitted serum for neural antibody testing at London Health Sciences Centre to assess for a possible autoimmune seizure etiology. While neural antibodies associated with younger-onset chronic temporal lobe seizures such as anti-GAD65 were not detected, mouse tissue indirect immunofluorescence (TIIF) showed staining compatible with anti-IgLON5 that was confirmed by cell-based assay (CBA) (Fig. 1).
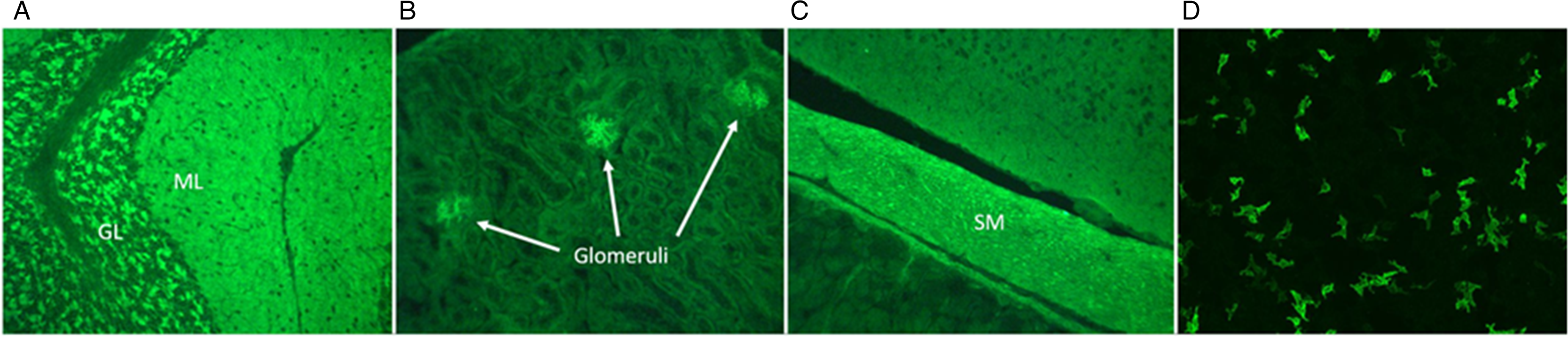
Figure 1: Detection of anti-IgLON5 in our patient. Mouse tissue indirect immunofluorescence shows staining compatible with anti-IgLON5, with prominent staining of the cerebellum (A), renal glomeruli (B), and intestinal smooth muscle (C). Confirmatory testing for anti-IgLON5 by EUROIMMUN cell-based assay is positive (D). GL = granular layer; ML = molecular layer; SM = smooth muscle.
Because of the positive neural antibody result, she was referred to our Autoimmune Neurology clinic for evaluation of seizures secondary to anti-IgLON5 disease. While she had seizures dating back 18 years, on targeted questioning she also reported sleep disturbances that had only developed over the last 2 years. She had not thought to mention these previously because sleep symptoms were mild. Specifically, she described insomnia, and her partner noted that she had increased snoring and movements during sleep. She reported no other significant cognitive, gait, speech, swallowing, or breathing difficulties. Neurologic examination was unremarkable. To further evaluate for autoimmune neurologic disease, lumbar puncture was performed that showed a normal cerebrospinal fluid (CSF) white blood cell count, mildly elevated protein, normal glucose, and no oligoclonal bands. Neural antibody testing of CSF also demonstrated positivity for anti-IgLON5 by TIIF and CBA. Testing for HLA alleles associated with anti-IgLON5 disease was negative for HLA-DRB1*10:01 but positive for HLA-DQB1*05:01.
Despite her reported sleep disturbances, video-polysomnography (V-PSG) as part of sleep consultation performed in the community was reportedly unremarkable. The patient underwent a 6-week trial of weekly intravenous methylprednisolone, with no reported improvement in seizure frequency or sleep disturbances. Given persistent sleep disturbances, she was referred to an academic center for a second sleep consultation. Repeat V-PSG at this center revealed mild abnormalities including minimal upper airway resistance syndrome, arousals from slow wave sleep without overt motor behavior, unusually prominent beta electroencephalographic activity in rapid eye movement (REM) sleep, and excessive fragmentary myoclonus (Fig. 2), potentially compatible with mild anti-IgLON5 disease. Axial muscle tone was normal in REM sleep, and NREM, REM, and wake states were clearly distinguishable.
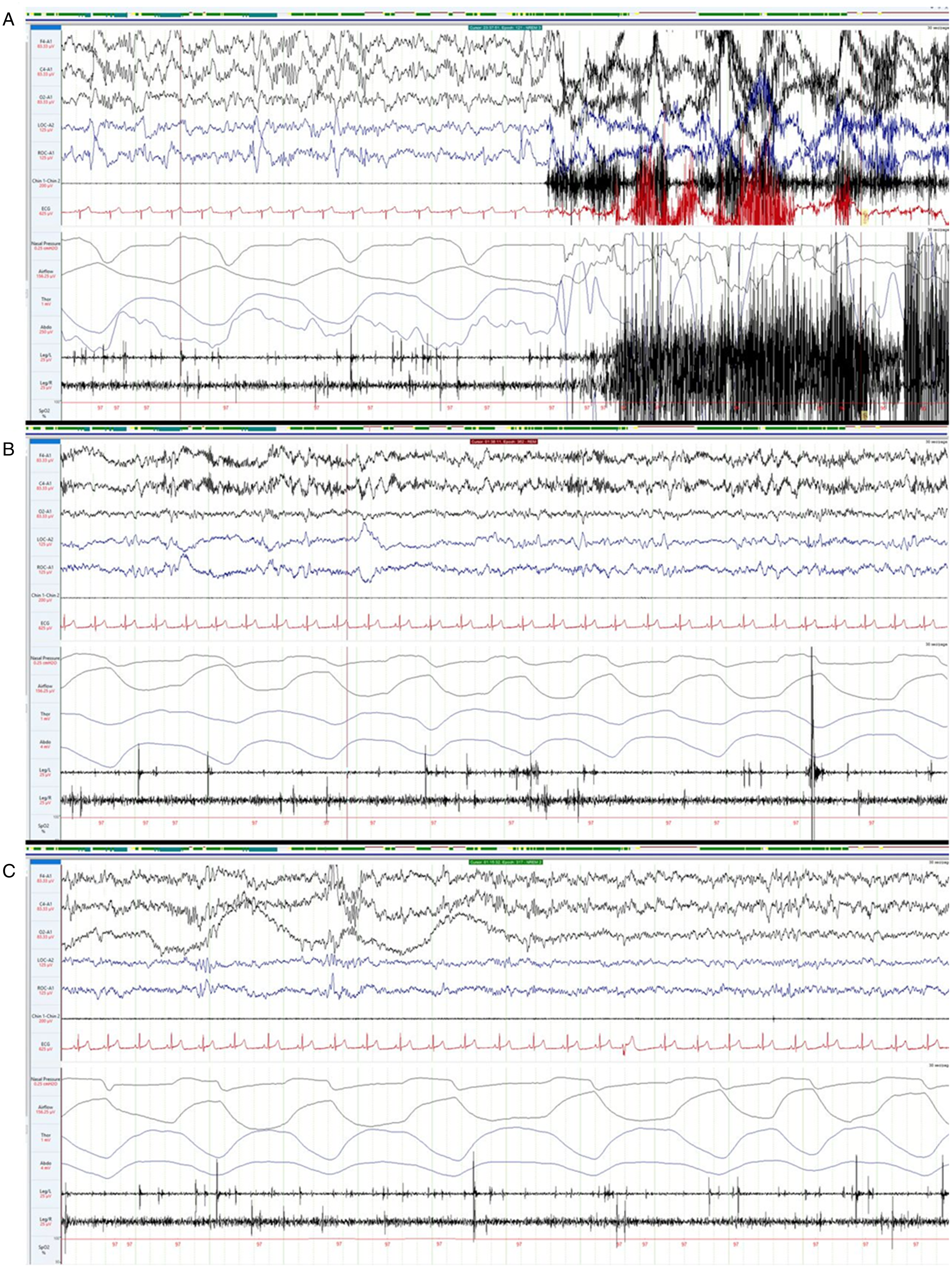
Figure 2: Video polysomnography findings in our patient. Video polysomnography shows mild abnormalities including arousals from slow wave sleep (A), unusually prominent beta electroencephalographic activity in REM sleep (B), and excessive fragmentary myoclonus (C).
In discussion with the patient, given the mildness of her sleep symptoms, we have opted to continue monitoring clinically and with serial polysomnography. If there is demonstrable evidence of progression compatible with anti-IgLON5 disease on follow-up, consideration will be given to further immunotherapy.
Anti-IgLON5 is a biomarker of a neurologic disease associated with prominent sleep disturbance. Reference Sabater, Gaig and Gelpi1 In addition to parasomnias and sleep-disordered breathing, other characteristic clinical manifestations of anti-IgLON5 disease that have been reported include gait impairment, bulbar dysfunction, oculomotor dysfunction, cognitive impairment, chorea, nervous system hyperexcitability, and dysautonomia. Reference Gaig, Graus and Compta2,Reference Honorat, Komorowski and Josephs3 Recently, seizures have also been reported to be a rare manifestation of anti-IgLON5 disease. Reference Wang, Wang and Zhao4 However, chronic temporal lobe seizures, with onset as young as observed in our patient and occurring in isolation for over a decade prior to the development of other symptoms more typical of anti-IgLON5 disease, have not been described. We therefore hypothesize that detection of anti-IgLON5 was incidental in our patient with likely idiopathic temporal lobe epilepsy, rather than indicative that younger-onset chronic temporal lobe seizures are a novel manifestation of anti-IgLON5 disease. Making this distinction is essential to minimize risk of “phenotype creep”, whereby the neurologic features of alternative diagnoses (e.g. idiopathic temporal lobe epilepsy) are mistakenly assumed to broaden the clinical spectrum of a neural antibody (e.g. anti-IgLON5) Reference Budhram, Mills, Shouman, Dyck, Hassan and Zalewski5 . Mistakenly attributing younger-onset chronic temporal lobe seizures to anti-IgLON5 disease could lead to unnecessary neural antibody testing in patients with epilepsy who otherwise lack a testing indication, which would adversely impact test utilization and lower test positive predictive value. Reference Chang, Nouri, Mirsattari, Burneo and Budhram6,Reference Budhram, Yang, Bhayana, Mills and Dubey7 In Autoimmune Neurology, concern for “phenotype creep” primarily arises in the context of false-positive antibody results generated by imperfectly specific assays among patients with diagnoses other than neural antibody-associated disease. Reference Budhram, Mills, Shouman, Dyck, Hassan and Zalewski5 Our case is unique, however, in that neural antibody detection by two assays (TIIF and CBA) in both serum and CSF has virtually perfect specificity, strongly arguing against a false-positive anti-IgLON5 result in our patient despite the atypical disease phenotype that prompted testing. Reference Budhram, Yang, Bhayana, Mills and Dubey7 The expectedly high specificity of the antibody result motivated targeted questioning pertaining to characteristic manifestations of anti-IgLON5 disease in our patient, which revealed that she had developed sleep disturbances in recent years. The mildness of sleep symptoms and V-PSG abnormalities in our case may be reflective of mild anti-IgLON5 disease, which was only incidentally diagnosed as part of the evaluation for a possible autoimmune seizure etiology.
Diagnosing a patient with mild anti-IgLON5 disease poses a therapeutic predicament. Unlike most neural antibody-associated diseases that present subacutely, patients with anti-IgLON5 disease may have insidious progression over years. Reference Nissen and Blaabjerg8 It is therefore possible that there are patients with particularly indolent, milder forms of anti-IgLON5 disease who go undiagnosed, and for whom the natural history of disease is unknown. This takes on particular importance when one attempts to weigh the potential benefits of immunotherapy and high reported mortality of anti-IgLON5 disease, Reference Honorat, Komorowski and Josephs3,Reference Nissen and Blaabjerg8 against the potential risks of immunotherapy and mildness of symptoms in a patient such as ours. In discussion with the patient, we have opted for ongoing monitoring with consideration of further immunotherapy if there is evidence of progression compatible with anti-IgLON5 disease, as a means of balancing risks and benefits of pursuing potentially long-term immunotherapy in her uniquely challenging case.
We report a diagnosis of mild anti-IgLON5 disease that was incidentally made in a patient with younger-onset temporal lobe seizures, highlighting the importance of a thoughtful approach to unexpected neural antibody results that are encountered in clinical practice. Additional study into the natural history of anti-IgLON5 disease is needed to determine the optimal management of patients with mild symptoms.
Data Access Statement
De-identified patient data are available to any qualified investigator upon reasonable request.
Statement of Authorship
SA: design and conceptualization, drafting of the manuscript.
BJM: design and conceptualization, critical revision of the manuscript for intellectual content.
AB: design and conceptualization, drafting of the manuscript.
Disclosure
Samir Alkabie reports no disclosures relevant to the manuscript. Brian J. Murray reports no disclosures relevant to the manuscript. Adrian Budhram reports that he holds the London Health Sciences Centre and London Health Sciences Foundation Chair in Neural Antibody Testing for Neuro-Inflammatory Diseases, and receives support from the Opportunities Fund of the Academic Health Sciences Centre Alternative Funding Plan of the Academic Medical Organization of Southwestern Ontario (AMOSO).




