Duchenne muscular dystrophy is an X-linked recessive disorder characterised by progressive skeletal and cardiac muscle dysfunction. Skeletal and respiratory muscle weakness typically becomes significant during the second decade of life. Reference Landfeldt, Thompson, Sejersen, McMillan, Kirschner and Lochmuller1 Early introduction of glucocorticoid therapy has delayed but not stopped the decline of skeletal and respiratory muscle function. Reference McDonald, Henricson and Abresch2,Reference Sawnani, Horn and Wong3 Glucocorticoid therapy in conjunction with early introduction of cough assist and positive pressure ventilation have fundamentally changed the “natural history” of Duchenne muscular dystrophy, and life expectancy now extends into the third to fourth decade of life. Reference Landfeldt, Thompson, Sejersen, McMillan, Kirschner and Lochmuller1 Improved long-term survival has underscored the importance of longitudinal, multidisciplinary care to manage the long-term comorbidities associated with progressive skeletal and cardiac muscle disease as well as the side effects of long-term glucocorticoid therapy. Reference Birnkrant, Bushby and Bann4
Recent case series have begun to document the presence of lower extremity oedema in patients with neuromuscular disease. Reference Greene and Sudduth5 Patients with Duchenne muscular dystrophy are potentially at heightened risk for the development of oedema given their loss of ambulation and the increasing frequency of positive pressure ventilation, obesity, and cardiomyopathy that occurs with age as each of these factors have been shown to contribute to the development of lower extremity oedema. Reference Scallan, Zawieja, Castorena-Gonzalez and Davis6–Reference Greene, Grant and Slavin8 This study sought to assess the frequency of lower extremity oedema in patients with Duchenne muscular dystrophy, the association with clinical status, and to describe home therapeutic measures currently being employed by patients and families.
Materials and methods
All Duchenne muscular dystrophy patients aged 15 years or older followed by the Comprehensive Neuromuscular Center at Cincinnati Children’s Hospital Medical Center and seen for at least one clinical visit between July, 2016 and July, 2019 were approached for the study between September, 2019 and May, 2020. The Duchenne muscular dystrophy clinical phenotype was determined either by DNA testing or lack of dystrophin on muscle biopsy. No monetary or alternative incentives were provided. The study was approved by the Cincinnati Children’s Hospital Medical Center Institutional Review Board, and the requirement to obtain documentation of informed consent was waived. Patients were given an oedema questionnaire in order to assess patient-reported oedema and therapeutic measures employed if oedema was present. A paper version of the survey was completed for patients screened and subsequently enrolled during clinic visits, while mail and e-mail-based surveys were distributed for patients who did not have a visit within the enrolment period. The e-mail was distributed based on family/patient provided e-mail addresses. The e-mail-based questionnaire contained a link to an online, HIPAA compliant, REDCap questionnaire which prevented duplicate entries and allowed for the linkage of survey results and clinical findings. A total of 188 patients were screened and 52 completed the survey and had an available clinical visit within a year that could be linked in order to assess potential risk factors for the development of oedema. The median time between clinical exam and survey completion was 1 day (range 0–208 days). Ten patients had > 1 month between survey completion and the preceding clinical visit which was used to assess clinical status (as noted below) with a time between exam and survey completion of 42, 78, 92, 104, 112, 113, 154, 186, 202, and 208 days, respectively.
Clinical status including demographics, medication use, ambulatory status, forced vital capacity, ventilator support (and reported adherence with ventilator support), left ventricular ejection fraction, and documentation of oedema were obtained by chart review from the patient’s most recent preceding clinical visit (i.e., clinical values were not assessed in the questionnaire). Ambulatory status including date of loss of ambulation is routinely reported in our centre’s neurology clinic visits. The exact date of loss of ambulation was used where available, for example, when a patient lost ambulation after a fall or fracture. Among patients without a specific date, the month of loss of ambulation was used. Clinically indicated pulmonary function testing was obtained as previously described at our centre. Reference Sawnani, Horn and Wong3 The patient’s most recent clinically obtained echocardiogram or cardiac magnetic resonance imaging was used to assess systolic function. Patient height was directly measured as standing height for ambulatory patients or calculated based on ulnar length as is commonly performed clinically for non-ambulatory patients. Reference Gauld, Kappers, Carlin and Robertson9 Photographic consent was obtained from a patient prior to use of a photo for this manuscript.
Oedema was considered present by clinician evaluation if documented by at least one clinical provider and was not limited to a specific specialty or provider type (e.g., physician, advanced practice nurse, and physical therapist). Among the patients who reported oedema on the questionnaire but were not noted to have oedema at the time of their most recent, preceding clinical encounter, the subsequent clinical encounter was used to confirm the presence of patient-reported oedema during the clinical exam (Fig 1). This follow-up encounter was examined to confirm that the questionnaire results were consistent with clinical exam to ensure patients were appropriately classified into oedema and no oedema groups. The follow-up encounter timing, specific provider visits, and diagnostic testing were all determined clinically as part of a patient’s standard of care and were not modified based on inclusion in the study or the results of the survey. All patients who reported clinical oedema were confirmed to have oedema at the time of subsequent exam. The results of the survey were not available to clinicians and thus did not guide the clinical exam at any visit. Four patients reported no oedema but were found to have oedema at the time of the provider exam. These patients were included in the oedema group for the purpose of the study. Differences in the severity of oedema by patient and providers were not assessed as the exam findings were collected retrospectively, and the grading of oedema was not standardised among providers.

Figure 1. Diagram depicting study results and categorisation of patients into groups with and without oedema based on patient survey results and clinical exam (the clinic visits were conducted independently of the survey results).
Statistical analysis
Statistical analyses were performed using SAS version 9.4 (Cary, North Carolina). Summary statistics are reported as number (frequency) or median (interquartile range) based on the nature of the variables. Comparisons were made between patients with and without oedema. Differences in proportion were assessed by X2 test. Comparisons of medians were made using Wilcoxon rank sum test for continuous variables. Predictors of the presence of the outcome of interest (oedema versus no oedema) were assessed using logistic regression. Potential predictors were assessed based on the initial intergroup comparisons. Predictors assessed included body mass index, deflazacort therapy, and duration since the loss of ambulation. Regression models were constructed to assess predictors of the presence of oedema, and variables with p-value ≤ 0.05 were included in the final model.
Results
Patient characteristics and oedema survey results
A total of 52 patients completed the oedema survey. Demographics, ambulatory status, and steroid therapy are listed in Table 1. Cardiac and pulmonary function and the associated therapies are listed in Table 2. The median age of patients was 20 years (17.6–21.9 years). The median height, weight, and body mass index were 152 cm (144–165 cm), 57.6 kg (50.1 kg–69.3 kg), and 27.2 (22.3–29.6), respectively. The majority were non-ambulatory (83%), and the median duration of time since the loss of ambulation was 5.9 years (1.9–9.1 years). Forty-nine patients were currently on steroid therapy (94%), two patients (4%) were not currently on steroids but were historically, and 1 patient (2%) was steroid-naïve. The majority of patients were on deflazacort (41, 79%), and the median duration of steroid therapy was 12.8 years (10.8–15.2 years).
Table 1. Patient demographics, ambulatory status, and steroid therapy. Values are reported as number (frequency) or median (interquartile range [IQR])

Table 2. Cardiac and pulmonary function and current clinical therapies

BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; FVC, forced vital capacity.
The median left ventricular ejection fraction was 55% (49%–59%) (Table 2). The majority of patients had an ejection fraction assessed by cardiac magnetic resonance imaging (32, 62%). Echocardiography was used in the remaining patients. Among those patients, all but two (4%) had adequate images for calculating the ejection fraction. The two patients with inadequate images had qualitatively normal and moderate systolic dysfunction, respectively. Forty-eight patients (92%) were on angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blocker (ARB), or angiotensin receptor II blocker – neprilysin inhibitor therapy (ARNI) therapy. Forty patients (77%) were on mineralocorticoid antagonist therapy and 35 (67%) patients were on beta-blocker therapy. The median forced vital capacity and forced vital capacity % predicted for the total cohort were 1.9L (1.36–2.52 L) and 50% (39%–75%) predicted, respectively. Forty-one patients (79%) were prescribed respiratory support, although only 29 patients (57%) report consistent use.
Lower extremity oedema
Oedema was common (Fig 1) as twenty-four patients (46%) reported some degree of oedema (Tables 1 and 2). Twelve of the 24 patients (50%) who reported oedema indicated swelling extended beyond the feet and up to the knees. Of the patients with oedema, 16 (67%) indicated their swelling was mild based on a provided scale (Supplement 1) and that they had no sores, skin breakdown, or infections of their feet or below their knees. Two patients with oedema (8%) reported sores, skin breakdown, or infections of their feet or below their knees. Figure 2 depicts an example of cellulitis in an adult with Duchenne muscular dystrophy and with normal biventricular systolic function and a history of bilateral pitting oedema to mid-calf. The majority of the patients with oedema (15, 63%) reported using no therapy for their oedema, four (17%) utilised compression stockings, and one (4%) reported manual massage. There was discrepancy between patient-reported oedema and provider-documented oedema at the time of the initial encounter and survey response (Fig 1). Fourteen of 20 patients (70%) with self-reported oedema had no provider-documented oedema at the time of initial encounter. All of these patients were noted to have oedema at their subsequent encounter. A small number of patients (4/32) who reported no oedema were noted to have oedema on exam.
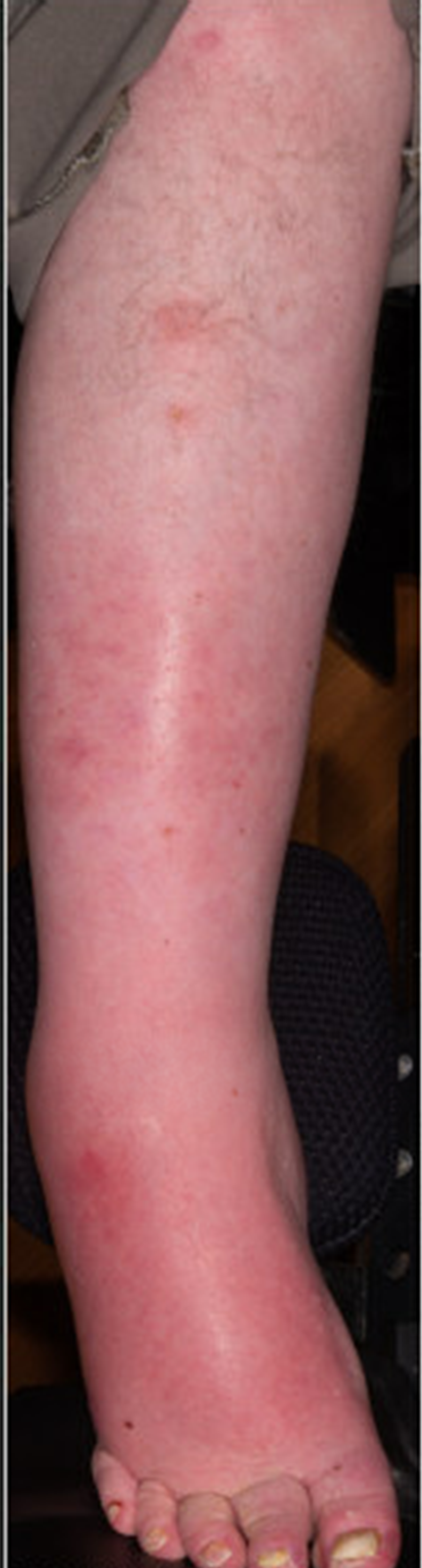
Figure 2. Infection of the legs in a 35-year-old Duchenne patient with a history of bilateral pitting oedema. The degree of oedema noted in the photograph is baseline, and the erythema was new at the time of infection. Photographic consent was obtained from the patient prior to use of the photo for this manuscript.
Comparing patients with and without oedema
There was no significant difference in age (19.9 years versus 20 years; p = 0.76) or height (152 cm versus 153 cm; p = 0.8) between groups; however, patients with oedema had greater weight (60.4 kg versus 54.4 kg; p = 0.05) and this translated to a greater body mass index (28.3 versus 24.8; p = 0.01). Patients with oedema were less frequently treated with deflazacort and more commonly treated with prednisone/prednisolone (p = 0.05). There was no significant intergroup difference in the duration of glucocorticoid therapy (12 years versus 13 years; p = 0.65). Patients with oedema also had a greater duration of loss of ambulation (8.4 years versus 3.5 years; p = 0.004). There was no significant difference in left ventricular ejection fraction (55% versus 55%; p = 0.86) or cardiac medications between groups (all p > 0.05) (Table 2). There was no significant difference in force vital capacity, force vital capacity % predicted, or non-invasive ventilator support used between groups (all p > 0.05) (Table 2).
Predictors of oedema
Independent predictors of the presence of oedema included body mass index (p = 0.009) and duration of loss of ambulation (p = 0.02) on multivariate analysis. Deflazacort use (p = 0.59) was not associated with difference in oedema on multivariate analysis.
Discussion
This study sought to describe the frequency, severity, therapeutic approach, and predictors of lower extremity oedema in Duchenne muscular dystrophy. The current study suggests that in adolescent and adult patients, oedema is prevalent, increases with time since the loss of ambulation, and is associated with greater body mass index. Furthermore, oedema often occurs in many patients when systolic function is preserved. Infection and skin breakdown are also relatively frequent among teenagers and young adults with oedema suggesting this may be a potential source of infection in the boys and young men with Duchenne muscular dystrophy. Importantly, the survey also discovered that while patient-reported oedema is common, both providers and patient/caregivers may miss the presence of oedema unless this is specifically assessed.
Heart failure, obesity, and loss of ambulation have been identified as contributors of lower extremity oedema in non-Duchenne muscular dystrophy populations. Reference Scallan, Zawieja, Castorena-Gonzalez and Davis6,Reference Rabe, Partsch and Hafner10–Reference Urbanek, Jusko and Kuczmik13 Understanding the factors that contribute to lower extremity oedema in Duchenne muscular dystrophy will be increasingly important as the number of patients with Duchenne muscular dystrophy surviving into the third and fourth decades of life continues to increase given the hazard for each of these factors increases with age. The current study specifically identified greater body mass index and greater duration of loss of ambulation as predictors of oedema in a cohort of Duchenne muscular dystrophy patients predominately in their late teens and twenties and with relatively preserved systolic function. The association between increased body mass index and increased frequency of oedema is especially notable, given it is a potentially modifiable risk factor, independent of age and duration time since the loss of ambulation. This study also adds to data documenting the long-term morbidity associated with increased body mass index and adiposity in teens and men with Duchenne muscular dystrophy including glucose intolerance, earlier cardiac dysfunction, and decreased respiratory strength. Reference Henson, Lang and Khoury14–Reference Chew, Carey, Ho, Mallitt, Widger and Farrar16
Obesity is a known risk factor for the development of oedema in non-Duchenne muscular dystrophy patients. When compared to lean controls, overweight and obese adolescents have 120% and > 400% risk of lower extremity oedema, respectively. Reference Inge, King and Jenkins17 Among adults, obesity has been identified as a risk factor for both the development of oedema and for subsequent cellulitis and hospitalisation. Reference Greene, Grant and Slavin8,Reference Burian, Karlsmark, Franks, Keeley, Quéré and Moffatt18 The causes of oedema in obesity are likely multi-factorial including structural lymphatic abnormalities, elevated venous pressures related to intra-abdominal pressures, and venous insufficiency. Reference Vasileiou, Bull, Kitou, Alexiadou, Garvie and Coppack19 It remains unclear how each of these factors relate to Duchenne muscular dystrophy, and what interplay occurs with oedema related to oedema as a result of limited mobility, which contributes to oedema in other neuromuscular disorders. Reference Greene and Sudduth5
While left ventricular dysfunction was not identified as a predictor of oedema in the current study, the cohort was comprised of patients with preserved or relatively preserved systolic function. Thus, we cannot rule out the cardiac contribution of lower extremity oedema in patients with greater degrees of systolic dysfunction, especially those with biventricular dysfunction which tends to be a late finding as respiratory and cardiac disease progress, or the role of diastolic dysfunction. Reference Mehmood, Ambach and Taylor20 In fact, in clinical practice, we have observed heart failure-related oedema on numerous occasions as heart failure progresses as in other populations. The fact that oedema occurs independent of significant left ventricular systolic dysfunction or clinical heart failure is also important as early signs of heart failure-related oedema may be overlooked due to pre-existing, non-cardiac-related lower extremity oedema. The interplay between non-cardiac and cardiac-driven oedema would also be relevant when discussing potential therapy. Compression stockings, manual lymphatic drainage, and pneumatic compression devices are commonly used as non-invasive interventions for the treatment of venous and lymphatic oedema. However, the use of compression stockings and pneumatic compression devices can result in a transient increase in venous return to the right heart leading to acute increases in right atrial and pulmonary artery pressures in patients with heart failure that may or may not be well tolerated. Reference Rabe, Partsch and Hafner10,Reference Nose, Murata and Wada11,Reference Urbanek, Jusko and Kuczmik13 Overall, data are lacking regarding the use of compressive therapies in managing oedema in Duchenne muscular dystrophy, and thus the initiation of therapy should be performed in conjunction with cardiac consultation among patients with appreciable cardiac dysfunction.
Finally, it is worth noting that at the onset of the study, providers often did not observe or appreciate patient-reported oedema. While it is possible this may reflect daily variations in oedema, the fact that all patients who reported oedema but were noted to have no oedema on the first provider exam were noted to have oedema on subsequent exams suggests this was more likely due to provider oversight. Following the results of this survey and increasing morbidity in older patients (as noted in Fig 2), our clinic has become more cognizant of the impact of oedema on clinical outcomes, and thus exams have become more comprehensive. The impact of this clinical change on interventions and outcomes will need to be demonstrated with time.
Conclusion
In conclusion, the current study suggests that routine assessment of lower extremity oedema is indicated in patients with Duchenne muscular dystrophy. This study also identifies maintaining healthy body size as a potential therapeutic target in minimising the risk for oedema. Multidisciplinary care will be required to understand the mechanisms of oedema in the larger cohort of patients with Duchenne muscular dystrophy, especially among those with more advanced cardiac disease and long-term positive pressure ventilation which may exacerbate the problem.
Limitations
The current study focused on generalised descriptions of oedema. Consistent assessments of disease severity will be required to reliably assess the severity of oedema, the impact of oedema on long-term outcomes, and the variation across the full spectrum of disease severity in Duchenne muscular dystrophy. The current study also reports discrepancy between patient and provider assessments of the presence of oedema. Some of the differences could be due to the time elapsed between questionnaire completion and clinical exam, and during that interval, a given patient’s oedema status changed. However, we believe that appreciable changes in clinical status are unlikely given the small amount of elapsed time between questionnaire completion and exam but acknowledge this could only be addressed with coincident questionnaires and exams in the full cohort.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122001342.
Acknowledgements
The authors thank Beth Dupont, RN, and Care Manager at Cincinnati Children’s Hospital Medical Center, for assisting with collecting surveys during patient clinical visits. The authors are also grateful for the resources provided by the Heart Institute Research Core within Cincinnati Children’s Hospital Medical Center. There is no conflict of interest or funding provided by Beth or Heart Institute Research Core.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.







