Children with CHD are at risk for increased pulmonary vascular resistance following cardiac surgery with cardiopulmonary bypass Reference Lindberg, Olsson, Jögi and Jonmarker1 ; 2–16% of these children will experience post-operative pulmonary hypertension, which can produce severe haemodynamic consequences. Reference Lindberg, Olsson, Jögi and Jonmarker1–Reference Bando, Turrentine and Sharp3 Complications from pulmonary hypertension are one of the leading causes of post-operative mortality. Reference Schlingmann, Thiagarajan and Gauvreau4
Cardiopulmonary bypass and intra-operative circulatory arrest contribute to post-operative pulmonary hypertension though complement activation, excess thromboxane and endothelin production, microthrombi development, and inhibition of endogenous nitric oxide release, which together result in endothelial cell dysfunction, increased pulmonary vascular reactivity, vasoconstriction, and increased pulmonary vascular resistance. Reference Adatia and Beghetti5–Reference Atz and Wessel8 Furthermore, volume and pressure loading of the pulmonary arteries from left-right shunt lesions, vascular congestion from systemic ventricular dysfunction or anatomic strictures, or underlying pulmonary vascular disease can lead to pre-operative pulmonary hypertension, which places children at additional risk for haemodynamically significant post-operative pulmonary hypertension. Reference Bando, Turrentine and Sharp3,Reference Schlingmann, Thiagarajan and Gauvreau4,Reference Kozlik-Feldmann, Hansmann, Bonnet, Schranz, Apitz and Michel-Behnke9,Reference Hansmann10 Haemodynamically significant pulmonary hypertension can lead to pulmonary hypertensive crises, consisting of acute elevations in pulmonary artery resistance leading to right ventricular failure, hypoxia, and systemic hypotension, which can be especially detrimental in the post-operative period. Reference Bando, Turrentine and Sharp3,Reference Schlingmann, Thiagarajan and Gauvreau4
Reducing the incidence and mortality of post-operative pulmonary hypertension is critical and requires early recognition and intervention through careful treatment of hypoxia and acidosis, as well as management of analgesia and sedation. Reference Adatia and Beghetti5,Reference Brunner, de Jesus Perez and Richter6 To optimise haemodynamics, pulmonary vasodilator therapies are often used in the post-operative setting. Reference Hansmann, Koestenberger and Alastalo11 These inhaled or systemic medications aim to vasodilate the pulmonary vasculature to improve pulmonary blood flow, reduce right ventricular afterload, and maintain left ventricular cardiac output. No drugs are currently labelled by the Food and Drug Administration for treatment of post-operative pulmonary hypertension or acute pulmonary hypertensive crises. 12–16 This review aims to assess the current literature of pulmonary vasodilator medications in the post-operative setting, specifically to understand optimal treatment, dose, timing, and patient population, in order to define areas for further research and improve post-operative outcomes in children with CHD.
Materials and methods
Search strategy
Similar to previously described, PubMed and EMBASE were searched to identify studies investigating the use of pulmonary vasodilator medications in children following cardiac surgery with cardiopulmonary bypass. Reference King, Thompson and Foote17–Reference Foote, Hornik, Hill, Rotta, Chamberlain and Thompson19 Studies from 2000 through 2020 were included to assess medication use in current clinical practice. Children were defined as from birth to age 18 years. The search terms “postoperative care,” “heart surgery,” “cardiopulmonary bypass,” “pediatric,” “pulmonary vasodilator,” “vasodilator,” “vasorelaxant,” “vasoactive antagonist,” “vasodilatant,” “vasodilating,” and “vasodilative” were used to generate an initial group of studies. The last search was performed on 5 May, 2021. Animal studies, studies in languages other than English, and studies focused on pre- or intra-operative medication use were excluded. Case reports, letters, editorials, and comments were also excluded. Search strategies are shown in the Appendix. A total of 271 studies were initially identified.
Study selection
Identified studies were imported into EndNote (Version X9, Clarivate Analytics, Philadelphia, PA, USA). Two reviewers independently screened and reviewed study abstracts and titles. Studies were eligible for inclusion if the primary focus was pulmonary vasodilator administration in the postoperative period for children following cardiac surgery with cardiopulmonary bypass. The full article was then reviewed to ensure appropriateness prior to data extraction.
Data extraction and synthesis
A standardised data collection form was used to extract the relevant data from each eligible study. The following data were collected: study characteristics (including study design and years of study), study population characteristics (including age and cardiac defects), intervention (including medication administered and the presence and type of control used), study endpoints, and results. For each medication, the dose, route and timing of administration, primary outcome, and secondary outcomes were compiled and analysed. Search strategies, study inclusion and exclusion criteria, and the standardised data collection form were all prespecified prior to data analysis. We did not assess for risk of bias. Given the heterogeneity of study outcomes, we compared studies qualitatively without performing a quantitative meta-analysis of outcomes or bias across studies. This review followed the guidelines for reporting systematic reviews as outlined by Liberati et al. Reference Liberati, Altman and Tetzlaff20
Results
A total of 26 studies in 42,971 children across four medication classes met inclusion criteria as shown in Figure 1. Study characteristics are summarised in Table 1. Three studies were multi-centre and 23 were single centre. The majority of patients (41,872) came from one multi-centre registry study. Reference Wong, Loomba, Evey, Bronicki and Flores25 Twelve studies were retrospective and 14 were prospective. Eleven of the prospective studies involved randomisation: four studies had a randomised control arm and seven studies were randomised between therapies. Reference Göthberg and Edberg21–Reference Morris, Beghetti, Petros, Adatia and Bohn23,Reference Stocker, Penny, Brizard, Cochrane, Soto and Shekerdemian31–Reference Limsuwan, Wanitkul, Khosithset, Attanavanich and Samankatiwat34,Reference Xu, Zhu, Liu, Gong, Gattrell and Liu36–Reference Peiravian, Amirghofran, Borzouee, Ajami, Sabri and Kolaee38,Reference Fraisse, Butrous, Taylor, Oakes, Dilleen and Wessel41,Reference Mendoza, Albert and Belda43,Reference Farah, Ahmad-Ali, Hanane and Abbas44,Reference Hill, Maharaj, Li, Thompson, Barker and Hornik46 In terms of primary outcomes, 14 studies assessed change in pulmonary pressures Reference Göthberg and Edberg21,Reference Morris, Beghetti, Petros, Adatia and Bohn23,Reference Yoshimura, Yamaguchi and Oka28–Reference Cai, Su and Shi32,Reference Limsuwan, Wanitkul, Khosithset, Attanavanich and Samankatiwat34–Reference Xu, Zhu, Liu, Gong, Gattrell and Liu36,Reference Peiravian, Amirghofran, Borzouee, Ajami, Sabri and Kolaee38,Reference Nemoto, Sasaki and Ozawa40,Reference Giordano, Palma and Poli42,Reference Farah, Ahmad-Ali, Hanane and Abbas44 ; four assessed the incidence of pulmonary hypertensive crises, although definitions varied Reference Miller, Tang, Keech, Pigott, Beller and Celermajer22,Reference Loukanov, Bucsenez and Springer33,Reference Onan, Ozturk, Yildiz, Firat Altin, Odemis and Erek37,Reference Nemoto, Sasaki and Ozawa40 ; three evaluated the requirement for additional therapy for pulmonary hypertension Reference Riley, Mastropietro and Sassalos26,Reference Lee, Hillier and Knoderer39,Reference Fraisse, Butrous, Taylor, Oakes, Dilleen and Wessel41 ; and five used clinical endpoints including duration of pleural drainage (n = 3) or mechanical ventilation (n = 2), and mortality (n = 2). Reference Tominaga, Iwai and Yamauchi24,Reference Wong, Loomba, Evey, Bronicki and Flores25,Reference Journois, Baufreton, Mauriat, Pouard, Vouhé and Safran27,Reference Mendoza, Albert and Belda43,Reference Koski, Suominen, Raissadati, Knihtilä, Ojala and Salminen45 One included study evaluated the pharmacokinetics of the investigated drug, and three evaluated a dose–response curve. Reference Göthberg and Edberg21,Reference Morris, Beghetti, Petros, Adatia and Bohn23,Reference Xu, Zhu, Liu, Gong, Gattrell and Liu36,Reference Hill, Maharaj, Li, Thompson, Barker and Hornik46 Haemodynamic data included pulmonary arterial pressures as measured by direct pulmonary artery catheter in 13 studies, central venous pressures as measured by direct central venous catheter in six studies, atrial pressures as measured by direct atrial catheter in five studies, and right ventricular pressures as estimated from doppler of tricuspid regurgitation in two studies. Reference Göthberg and Edberg21–Reference Morris, Beghetti, Petros, Adatia and Bohn23,Reference Yoshimura, Yamaguchi and Oka28–Reference Peiravian, Amirghofran, Borzouee, Ajami, Sabri and Kolaee38,Reference Nemoto, Sasaki and Ozawa40–Reference Giordano, Palma and Poli42,Reference Farah, Ahmad-Ali, Hanane and Abbas44 Medications studied included inhaled nitric oxide [14/26]; the prostacyclin analogue, iloprost [5/26]; phosphodiesterase inhibitors (sildenafil [9/26] and milrinone [2/26]); and endothelin receptor antagonists (ambrisentan [1/26]). Inhaled nitric oxide was used as routine care or open-label rescue therapy in seven of the 12 studies in which it was not a primary intervention.
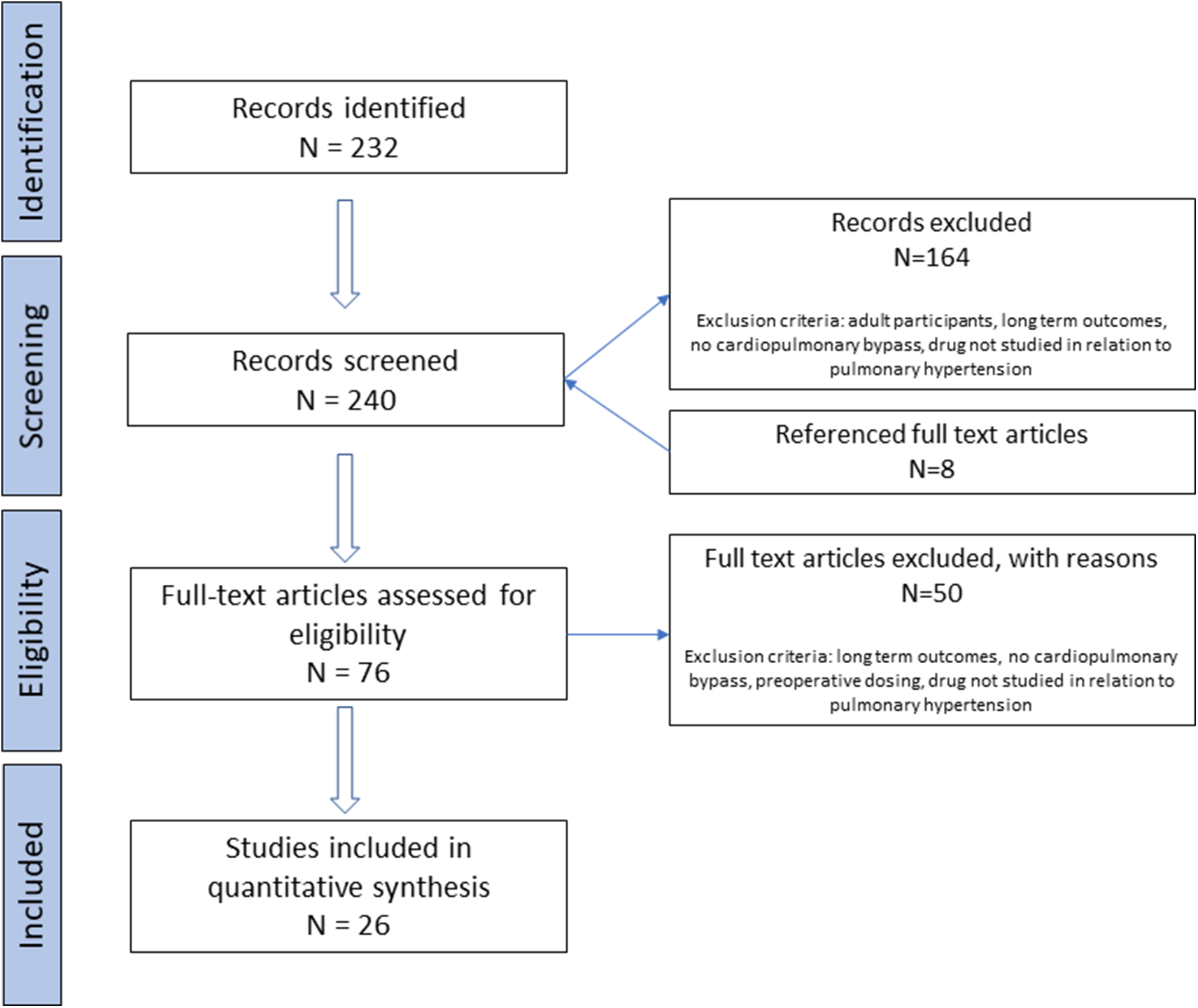
Figure 1. Flow diagram of study selection.
Table 1. Characteristics of included studies and study populations.

ASD, atrial septal defect; AVSD, atrioventricular septal defect; BDG, bidirectional Glenn; CPB, cardiopulmonary bypass; CVP, central venous pressure; HFNC, high-flow nasal canula; iNO, inhaled nitric oxide; PDE, phosphodiesterase; PaCO2, partial atrial pressure of carbon dioxide; PaO2, partial atrial pressure of oxygen; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PHTC, pulmonary hypertensive crisis; Ppm, parts per million; PVR, pulmonary vascular resistance; TAPVR, total anomalous pulmonary venous return; TCPC, total cavopulmonary connection; TPP, transpulmonary pressure; TR, tricuspid regurgitation; UOP, urine output.
Data presented as mean ± standard deviation [SD] or median [interquartile range].
Inhaled nitric oxide
Nitric oxide is produced endogenously by endothelial cells leading to the relaxation of smooth muscles via the conversion of guanosine triphosphate to cyclic guanosine monophosphate. Reference Palmer, Ferrige and Moncada47,Reference Ichinose, Roberts and Zapol48 When administered as an inhaled gas (inhaled nitric oxide), nitric oxide leads to pulmonary vasodilation in ventilated areas of the lung. Reference Frostell, Fratacci, Wain, Jones and Zapol49 Haemoglobin rapidly binds and inactivates nitric oxide, minimising its systemic vasodilator effects. Reference Palmer, Ferrige and Moncada47 Haemoglobin bound to nitric oxide is oxidised to methaemoglobin, which is then metabolised to nitrate. Reference Frostell, Fratacci, Wain, Jones and Zapol49 Methaemoglobinaemia is frequently monitored during inhaled nitric oxide administration, but rarely rises to clinically significant levels. Reference Steudel, Hurford and Zapol50 Withdrawal of inhaled nitric oxide is associated with decreased partial pressure of oxygen and rebound pulmonary hypertension within minutes of discontinuation, likely due to downregulation of endogenous nitric oxide production and decreased cyclic guanosine monophosphate. Reference Black, Heidersbach, McMullan, Bekker, Johengen and Fineman51 A gradual stepwise wean is recommended to limit this phenomenon. Reference Ichinose, Roberts and Zapol48,Reference Sokol, Fineberg, Wright and Ehrenkranz52 Additionally, inhaled nitric oxide is one of the most expensive medications routinely given to critically ill children, suggesting the need for judicious usage. Reference Campbell, Herbst, Briden, Neff, Ruscher and Hagadorn53
Inhaled nitric oxide has been used in infants and children with hypoxic respiratory failure for more than two decades. Inhaled nitric oxide increases extracorporeal membrane oxygenation-free survival in children with paediatric acute respiratory distress syndrome and decreases need for extracorporeal membrane oxygenation without a demonstrated mortality benefit in term and near-term neonates with hypoxic respiratory failure and persistent pulmonary hypertension. Reference Bronicki, Fortenberry, Schreiber, Checchia and Anas54–Reference Clark, Kueser and Walker56 Inhaled nitric oxide is Food and Drug Administration-labelled for neonates with pulmonary hypertension-related hypoxic respiratory failure, but its use in other clinical scenarios, including the post-operative setting, remains off-label. 12 Fourteen studies of inhaled nitric oxide met our inclusion criteria.
Three single centre prospective studies of 148 total children evaluated the haemodynamic effects of inhaled nitric oxide in children with post-operative pulmonary hypertension. Reference Göthberg and Edberg21–Reference Morris, Beghetti, Petros, Adatia and Bohn23 At initial doses ranging from 3 ppm to 10 ppm, inhaled nitric oxide was associated with significantly decreased mean pulmonary artery pressure and pulmonary vascular resistance, as measured by pulmonary artery catheter, and rate of pulmonary hypertensive crises (defined as a ratio of pulmonary to systemic artery pressure greater than 0.75). No dose–response relationship was seen with increasing doses of inhaled nitric oxide from 3 ppm to 80 ppm, and no difference in response was seen between therapy at 5 ppm and 40 ppm. Reference Göthberg and Edberg21,Reference Morris, Beghetti, Petros, Adatia and Bohn23
Five single centre retrospective studies of 179 total children investigated the impact of inhaled nitric oxide (starting dose ranging from 10 to 25 ppm) in children following specific surgical repairs. Reference Tominaga, Iwai and Yamauchi24,Reference Journois, Baufreton, Mauriat, Pouard, Vouhé and Safran27–Reference Georgiev, Latcheva, Pilossoff, Lazarov and Mitev30 Combining inhaled nitric oxide with high-flow nasal cannula therapy for children following Fontan operation significantly reduced duration of mechanical ventilation, pleural drainage time, and total hospital stay, regardless of preoperative haemodynamics. Reference Tominaga, Iwai and Yamauchi24 In children with elevated cavopulmonary pressures following bidirectional Glenn or Fontan operation, inhaled nitric oxide therapy resulted in significantly decreased cavopulmonary and transpulmonary pressure gradients. Reference Yoshimura, Yamaguchi and Oka28–Reference Georgiev, Latcheva, Pilossoff, Lazarov and Mitev30 Of note, each study varied in the definition of elevated pressures using both transpulmonary pressure gradient (>8 mmHg) and elevated cavopulmonary pressure (with threshold ranging from >15 to >20 mmHg). However, children with haemodynamically significant anatomic lesions requiring surgical reintervention (e.g., pulmonary artery thrombus or Glenn shunt stenosis) or those without baseline elevated pressures did not have a significant improvement, suggesting a therapeutic benefit only for children meeting certain haemodynamic criteria. Similarly, for children following surgical atrioventricular canal repair, therapy with inhaled nitric oxide was associated with improved mortality only for the sub-cohort of those who experienced pulmonary hypertensive crises (defined as pulmonary artery pressure >70% systemic arterial pressure with associated decreased venous or arterial oxygen saturation, as measured by either pulmonary arterial catheter or echocardiography. Reference Hill, Maharaj, Li, Thompson, Barker and Hornik46
Two multicentre retrospective studies of 42,088 total children compared outcomes between children who did and did not receive inhaled nitric oxide therapy. Reference Wong, Loomba, Evey, Bronicki and Flores25,Reference Riley, Mastropietro and Sassalos26 Inhaled nitric oxide usage was associated with increased intubation time, longer hospitalisation, increased cost of stay, and increased rate of mortality for children both with and without pulmonary hypertension, defined based on diagnosis codes. The strength of these studies is their multi-centre design allowing for analysis across a large cohort of children, but they are limited by an inability to assess indication for usage. They do not incorporate individual haemodynamics, ventilator settings, or inotrope requirements that may have confounded inhaled nitric oxide usage and outcomes.
Phosphodiesterase inhibitors
Phosphodiesterase-5 inhibitors
Intracellular phosphodiesterase degrades cyclic guanosine monophosphate, extinguishing its downstream vasodilator effects. Reference Ghofrani, Pepke-Zaba and Barbera57 Sildenafil is a phosphodiesterase-5 selective inhibitor that prevents cyclic guanosine monophosphate breakdown and leads to pulmonary vasodilation. Reference Weimann, Ullrich and Hromi58 Sildenafil is available in both oral and intravenous forms. Short-term oral therapy may reduce the risk for rebound pulmonary hypertension following inhaled nitric oxide wean in infants, and long-term treatment in children with World Health Organization Group I pulmonary hypertension (pulmonary arterial hypertension) improved functional class and haemodynamics compared to placebo therapy. Reference Humpl, Reyes, Erickson, Armano, Holtby and Adatia59–Reference Barst, Dunbar Ivy and Gaitan61 While commonly used, sildenafil therapy is associated with some controversy, due to the increased mortality risk with high doses seen in the Sildenafil in Treatment-Naive Children, Aged 1–17 Years, With Pulmonary Arterial Hypertension (STARTS) trials. Reference Barst, Beghetti and Pulido62 Although there may have been methodological limitations in this observed dose–outcome relationship, both European and Food and Drug Administration guidelines caution against higher doses and chronic use of sildenafil in children. Reference Hansmann, Koestenberger and Alastalo11,14,Reference McElhinney63 Side effects of short-term treatment include risk for systemic hypotension, headache, flushing, and gastrointestinal symptoms. Reference Simonca and Tulloh64 Nine studies of sildenafil met our inclusion criteria. Notably, all included studies were performed before the STARTS-2 trial results were made public.
Three studies of 159 total children with post-operative pulmonary hypertension found that treatment with sildenafil (oral starting dose 0.3–0.5 mg/kg with dosing frequency between every 3 and 6 hours; an intravenous continuous dose was selected to reach target plasma concentration of 40–360 ng/mL) was associated with significantly decreased pulmonary artery pressures and transpulmonary pressure gradient (as measured by pulmonary artery catheter or estimated by echocardiography), as well as duration of mechanical ventilation and ICU length of stay. Reference Peiravian, Amirghofran, Borzouee, Ajami, Sabri and Kolaee38,Reference Nemoto, Sasaki and Ozawa40,Reference Fraisse, Butrous, Taylor, Oakes, Dilleen and Wessel41 In two studies of 22 children, the addition of sildenafil (dosed at 0.3 mg/kg orally four times daily or 0.35 mg/kg IV as a single dose) to inhaled nitric oxide therapy at 20 ppm was associated with an additional reduction in pulmonary vascular resistance and a significant decrease in required inhaled nitric oxide dose. Reference Stocker, Penny, Brizard, Cochrane, Soto and Shekerdemian31,Reference Lee, Hillier and Knoderer39 However, three separate single-centre retrospective studies of 186 total children compared the effect of standardising treatment with sildenafil (starting doses range 2.1–4.6 mg/kg/day) for all children following Fontan operation with diverging results. Reference Giordano, Palma and Poli42,Reference Mendoza, Albert and Belda43,Reference Koski, Suominen, Raissadati, Knihtilä, Ojala and Salminen45 One study found sildenafil treatment to be associated with shorter duration of intubation, chest tube drainage, and ICU length of stay, but no difference in outcomes was seen in the other two studies and one suggested a more positive fluid balance on post-operative day one in children receiving sildenafil.
Phosphodiesterase-3 inhibitors
Milrinone is delivered intravenously and inhibits phosphodiesterase-3, which leads to vasodilation and increases inotropy through increased intracellular calcium, cyclic adenosine monophosphate, and cyclic guanosine monophosphate. Reference Hoffman, Wernovsky and Atz65,Reference Skoyles and Sherry66 Milrinone is the most used intravenous vasoactive medication for children with pulmonary hypertensive crises and may reduce pulmonary arterial pressures in children with pulmonary hypertension. Reference King, Thompson and Foote17,Reference Bernier, Romer and Bembea67 Nevertheless, its use in the paediatric population remains off-label. 15 Additionally, milrinone has a relatively long half-life compared to other inotropes and is primarily renally cleared, so both optimal dosing and timing of therapy in critically ill children at risk for acute kidney injury are important to define. Reference Larsson, Liedholm, Andersson, Keane and Henry68,Reference Hornik, Yogev and Mourani69 Two studies of milrinone met our inclusion criteria. Milrinone has demonstrated it can reduce post-operative low cardiac output syndrome in a multicenter, randomised, double-blind placebo control trial and is routinely used post-operatively to augment cardiac output. Reference Hoffman, Wernovsky and Atz65 While we focused on milrinone’s use as a pulmonary vasodilator or in patients with pulmonary hypertension as described in the inclusion criteria, the impact on pulmonary hypertension may be confounded by milrinone’s additional lusitropic and systemic vasodilatory effects.
In a single-centre randomised prospective trial, 48 children with both pre-operative pulmonary hypertension (based on echocardiographic tricuspid regurgitation gradient >30 mmHg) and moderate intraoperative pulmonary hypertension (defined as pulmonary artery to aortic pressure ratio 0.60–0.84, as assessed by pulmonary artery catheter) were started on either milrinone or sildenafil at time of cardiopulmonary bypass initiation. Treatment with milrinone dosed with 50 mcg/kg bolus followed by 0.75 mcg/kg/minute infusion compared favourably to oral sildenafil dosed 0.3 mg/kg every 3 hours with significantly lower pulmonary artery pressures and shorter ICU length of stay. Reference Farah, Ahmad-Ali, Hanane and Abbas44 In a separate single-centre prospective randomised trial of 46 children with increased pulmonary pressures following Fontan (central venous pressure of >15 mmHg or transpulmonary pressure >10 mmHg), therapy with either inhaled nitric oxide (at 20 ppm) or milrinone (at 0.5 mcg/kg/minute without loading dose) was associated with improved central venous and transpulmonary pressure gradient within 4 hours of therapy. Reference Cai, Su and Shi32 Combined therapy with both agents was associated with the most significant improvement in central venous and transpulmonary pressures, as well as significantly shorter intubation time. Together, these studies demonstrate that milrinone may be a useful therapeutic to augment the effect of other pulmonary vasodilators in children with post-operative pulmonary hypertension. However, a randomised controlled trial comparing milrinone to placebo as standard post-operative therapy for children following Fontan operation found no difference in length of hospital stay or other surrogate markers for efficacy, suggesting further work is needed to define the optimal population for treatment. Reference Costello, Dunbar-Masterson and Allan70
Prostacyclin analogues
Prostacyclin is produced by vascular endothelial cells as a metabolite of arachidonic acid and leads to vascular smooth muscle relaxation. Reference Mitchell, Ali, Bailey, Moreno and Harringon71 Intravenous infusions of prostacyclin rapidly decrease pulmonary vascular resistance, but carry risks for dose-limiting systemic side effects that include systemic hypotension, headache, and vomiting. Reference Rubin, Groves, Reeves, Frosolono, Handel and Cato72 Iloprost is a synthetic prostacyclin derivative that is available in both intravenous and aerosolised forms and is Food and Drug Administration approved for the long-term treatment of adults with World Health Organization Group I pulmonary hypertension. The use of iloprost in children or in peri-operative settings remains off-label. 13,Reference Lewis, Freed, Heymann, Roehl and Kensey73–Reference Olschewski, Simonneau and Galiè75 Five studies of iloprost met our inclusion criteria.
In a single-centre prospective study of eight children with post-operative pulmonary hypertensive crises (defined as systolic pulmonary artery pressure to systemic arterial pressure >0.6 as measured by pulmonary artery catheter with associated hypoxia), aerosolised iloprost (starting dose 0.5 mcg/kg over 10 minutes) was associated with a significant decrease in mean pulmonary artery pressure as assessed by pulmonary artery catheter and an increase in arterial oxygen saturation. Reference Limsuwan, Wanitkul, Khosithset, Attanavanich and Samankatiwat34 A single-centre retrospective study of seven infants with post-operative pulmonary hypertension found initiation of inhaled iloprost (1.25–5 mcg/dose administered every 2 hours) allowed inhaled nitric oxide therapy to be weaned off over a median of 5 hours without significant change in mean or systolic pulmonary artery pressures as assessed by pulmonary artery catheter. Reference Vorhies, Caruthers, Rosenberg, Yu and Gajarski35 Three single-centre prospective randomised trials with 52 total children evaluated the ability of iloprost to reduce pulmonary hypertensive crises. Reference Loukanov, Bucsenez and Springer33,Reference Xu, Zhu, Liu, Gong, Gattrell and Liu36,Reference Onan, Ozturk, Yildiz, Firat Altin, Odemis and Erek37 The definition of a hypertensive crisis varied across these studies based on the threshold of pulmonary artery to systemic arterial pressure ratio with associated hypotension or desaturation, and one study provided no definition. In infants with pulmonary hypertension, therapy with inhaled nitric oxide (10 ppm) or inhaled iloprost (0.5 mcg/dose) was associated with similar frequencies of pulmonary hypertensive crises. Compared to placebo, inhaled iloprost (dose 0.3–0.5 mcg/kg) significantly reduced the risk for pulmonary hypertensive crises in children with post-operative pulmonary hypertension. Nonetheless, in children with pre-operative pulmonary hypertension (defined as systolic pulmonary artery pressure >50 mmHg or mean pulmonary artery pressure >25 mmHg), initiation of intravenous iloprost (dosed at 2.0 ng/kg/minute) at time of cardiopulmonary bypass wean had no benefit in pulmonary pressures or in length of stay compared to placebo. Reference Onan, Ozturk, Yildiz, Firat Altin, Odemis and Erek37 These studies suggest that inhaled nitric oxide and iloprost may have similar haemodynamic effects, but further study will be needed to clarify the difference in outcomes between inhaled and intravenous iloprost, which may be related to patient population, route, or dose of drug.
Endothelin receptor antagonists
Endothelin receptor antagonists, including bosentan and ambrisentan, inhibit endothelin-1-mediated vasoconstriction leading to reduced pulmonary vascular resistance and reduced pulmonary artery pressures. Reference Hansmann10 Bosentan is Food and Drug Administration-labelled for the treatment of World Health Organization Group I pulmonary hypertension in children. 76 Pre-operative bosentan may improve pulmonary pressures prior to Fontan operation, and bosentan improved exercise capacity and functional status of children with Fontan physiology in a randomised trial Reference Hebert, Mikkelsen and Thilen77,Reference Hirono, Yoshimura and Taguchi78 ; however, bosentan is associated with hepatotoxicity, fluid retention, and anaemia that may be especially detrimental in the acute post-operative setting. Unlike bosentan, which is a non-selective endothelin receptor antagonist, ambrisentan selectively binds to endothelin-1 type A receptors and may reduce the risk for hepatotoxicity. Reference Aversa, Porter and Granton79 Preclinical study of ambrisentan in juvenile rats suggested a potential negative effect on brain weight. 16
Similar safety concerns in paediatric populations have not yet been demonstrated, but further safety data are needed, especially in younger children. Reference Ivy, Beghetti and Juaneda-Simian80 Use of ambrisentan is off-label in the paediatric population, but a single-centre prospective, randomised, placebo-controlled trial of 16 children demonstrated ambrisentan is overall safe in children immediately following Fontan operation. 16,Reference Hill, Maharaj, Li, Thompson, Barker and Hornik46 The study was primarily designed to assess the pharmacokinetics of ambrisentan in the post-Fontan setting. Three hours following treatment (2.5 mg daily for up to 3 days), children had significantly decreased plasma brain natriuretic peptide levels, Fontan pressures, and indexed pulmonary vascular resistance as assessed by central venous and atrial catheters. No benefit was seen compared to placebo in the clinical endpoints of chest tube output or length of hospitalisation, although the study enrolled only three placebo group patients and was, therefore, underpowered for detecting any difference. Further study will be needed to define the most beneficial timing and target population for endothelin receptor antagonists in the post-operative setting.
Discussion
Overall, we identified 26 studies of 42,971 children following cardiac surgery across four classes of pulmonary vasodilator medications over twenty years. Our review showed promising therapies to reduce postoperative pulmonary hypertensive crises, but more research is needed to identify the optimal dose, timing of initiation, and patient population for these therapies. Only three studies were multicentre, and of these, only one was a prospective trial. Although a relatively large number of studied children were identified across all studies, two retrospective studies of inhaled nitric oxide usage in 42,088 combined children comprised a substantial majority of the included children, and 20 of the 26 studies had fewer than fifty children. These small study sizes provide limited power to detect differences in more clinically significant outcomes such as duration of mechanical ventilation or hospitalisation and mortality. Accordingly, change in pulmonary pressures was the most common primary outcome (used in 14/26 studies), since with small studies it is easier to find significance with a surrogate marker of efficacy.
Single-centre trials of inhaled nitric oxide demonstrated improved pulmonary haemodynamics following drug initiation in some (children with severe pulmonary hypertension following atrioventricular canal repair or elevated pulmonary pressures following cavopulmonary connection), but not all (such as those with residual anatomic lesions or after cavopulmonary connection without significantly elevated pressures) subsets of post-operative children. Reference Göthberg and Edberg21,Reference Morris, Beghetti, Petros, Adatia and Bohn23,Reference Journois, Baufreton, Mauriat, Pouard, Vouhé and Safran27–Reference Georgiev, Latcheva, Pilossoff, Lazarov and Mitev30 Of note, only one of these studies had a clinical endpoint for efficacy (mortality), while all others assessed haemodynamic changes. Reference Journois, Baufreton, Mauriat, Pouard, Vouhé and Safran27 There was variation in studied inhaled nitric oxide dose, with 10 ppm and 20 ppm being the most commonly used starting doses. This variation makes the comparison of outcomes across studies challenging, although two included studies did not find a dose–response relationship with increasing inhaled nitric oxide doses from 3 ppm to 80 ppm, or 5 ppm to 40 ppm. Reference Göthberg and Edberg21,Reference Morris, Beghetti, Petros, Adatia and Bohn23
Similarly, oral sildenafil improved post-operative haemodynamics for some cohorts, including those with pre-operative pulmonary hypertension in a single-centre randomised controlled trial. Reference Onan, Ozturk, Yildiz, Firat Altin, Odemis and Erek37 Interestingly, three single-centre retrospective cohort studies found a divergent impact of sildenafil administration following Fontan operation, with only one suggesting improvements in intubation time and ICU length of stay, while two others showed no benefit and worse fluid balance. Reference Giordano, Palma and Poli42,Reference Mendoza, Albert and Belda43,Reference Koski, Suominen, Raissadati, Knihtilä, Ojala and Salminen45 These studies highlight the need for multicentre prospective trials to limit the impact of site-specific variation in care or changing protocols over time on study outcomes. Furthermore, there was a wide spectrum of sildenafil doses administered with mean daily dosing ranging from 1.2 mg/kg to 4.6 mg/kg, Reference Lee, Hillier and Knoderer39,Reference Mendoza, Albert and Belda43 making drawing conclusions across studies more challenging.
Furthermore, there was heterogeneity in the definition of haemodynamic outcomes. Five studies had a primary outcome or enrollment criteria of pulmonary hypertensive crisis, yet each used a different definition, varying in threshold pulmonary artery pressure to systemic arterial pressure ratio from >0.6 to >1.0 and with different requirements for associated hypotension or hypoxaemia. Reference Miller, Tang, Keech, Pigott, Beller and Celermajer22,Reference Loukanov, Bucsenez and Springer33,Reference Onan, Ozturk, Yildiz, Firat Altin, Odemis and Erek37,Reference Nemoto, Sasaki and Ozawa40 The modality of assessment of pulmonary pressures also varied between pulmonary artery catheter and echocardiogram across studies. Pulmonary artery catheters are considered the gold standard for assessing pulmonary pressures, while echocardiogram is less precise and not recommended to guide therapy alone in the outpatient setting. Reference Hansmann, Koestenberger and Alastalo11,Reference Augustine, Coates-Bradshaw and Willis81 Nevertheless, an echocardiogram may be a reasonable, less invasive technique in the post-operative setting where invasive pulmonary artery catheters are not routinely used. The variation in haemodynamic definitions also reflects the changing thresholds for pulmonary hypertension diagnosis, with recent lowering of threshold mean pulmonary artery pressure from ≥25 mmHg to ≥20 mmHg based on World Symposium of Pulmonary Hypertension 2018 guidelines. Reference Simonneau, Montani and Celermajer82 Standard and consistent criteria will be necessary to definitively determine which children will most benefit from medical therapy. Additionally, the heterogeneity of outcomes precluded combining the results of studies, performing quantitative meta-analysis of the included studies, or assessing the risk of bias in individual studies.
Despite the limitations of inhaled nitric oxide described earlier, in a recent survey of North American ICUs, all responding institutions reported inhaled nitric oxide usage for treatment of pulmonary hypertensive crises, although dosing ranges and weaning protocols were non-uniform. Reference Bernier, Romer and Bembea67 Two major drawbacks of inhaled nitric oxide therapy are the risk for rebound pulmonary hypertension, which may prolong weaning of respiratory support, and cost. Reference Sokol, Fineberg, Wright and Ehrenkranz52,Reference Campbell, Herbst, Briden, Neff, Ruscher and Hagadorn53 Three studies specifically mentioned the effects of inhaled nitric oxide withdrawal on haemodynamics: two studies of 28 total children found no significant effect, and one study of 47 children noted 17 had significant increases in central venous pressure and transpulmonary pressure gradient that required reinitiating therapy. Reference Morris, Beghetti, Petros, Adatia and Bohn23,Reference Yoshimura, Yamaguchi and Oka28,Reference Georgiev, Latcheva, Pilossoff, Lazarov and Mitev30 Successful earlier weaning from inhaled nitric oxide may be aided by transition to inhaled nitric oxide therapy delivered through high-flow nasal cannula or starting oral sildenafil prior to wean. Reference Tominaga, Iwai and Yamauchi24,Reference Lee, Hillier and Knoderer39 Aerosolized iloprost may have similar efficacy to inhaled nitric oxide at preventing pulmonary hypertensive crises and provide a viable, less expensive option for transitioning off inhaled nitric oxide therapy. Reference Loukanov, Bucsenez and Springer33,Reference Vorhies, Caruthers, Rosenberg, Yu and Gajarski35 Milrinone may augment the pulmonary vasodilatory effects of sildenafil and inhaled nitric oxide. Reference Cai, Su and Shi32,Reference Farah, Ahmad-Ali, Hanane and Abbas44
Early trials demonstrated the efficacy of inhaled nitric oxide to improve ECMO-free survival in neonates with pulmonary hypertension almost 25 years ago. 55 Multiple therapies for pulmonary hypertension have been developed since that time, and we identified studies across four different classes of medication. These allow for combination therapy either upfront or if initial monotherapy is not effective. Reference Hansmann, Koestenberger and Alastalo11 None of the included studies investigated combination therapy. Additional medications from the classes we have identified, such the phosphodiesterase 5 inhibitor tadalafil and prostacyclin epoprostenol have shown benefit in children with pulmonary arterial hypertension, but have not been well studied in the post-operative setting. Reference Ivy, Bonnet and Berger83,Reference Lammers, Hislop, Flynn and Haworth84 Novel medications such as the oral guanylate cyclase stimulant riociguat have shown clinical improvement in adult populations and represent potential options for investigation in children. Reference Rosenkrantz, Ghofrani and Beghetti85 Further study will be required to optimise these treatment strategies for children following cardiac surgery.
Overall, in single centre studies, children diagnosed with post-operative pulmonary hypertension by pulmonary artery catheter or echocardiogram had improved haemodynamics following initiation of pulmonary vasodilator therapy, yet definitive improvements in clinical endpoints such as intubation time, ICU length of stay, or mortality were not well demonstrated. Additionally, attempts to standardise to therapy for all children following a specific surgical repair, such as after Fontan operation, have largely been unsuccessful. This discrepancy in efficacy was demonstrated in two multicentre retrospective studies that found inhaled nitric oxide exposure overall was associated with increased intubation time, cost of therapy, and length of stay, even among children with a diagnosis of pulmonary hypertension. Reference Wong, Loomba, Evey, Bronicki and Flores25,Reference Riley, Mastropietro and Sassalos26 These multicentre analyses are limited by their inability to distinguish the haemodynamics of individual patients and may be selecting for sicker children that require inhaled nitric oxide exposure; however, this variation in outcomes demonstrates the knowledge gap that exists in this population. Additionally, of the included studies, 14 assessed therapies for only children with biventricular repair, nine assessed therapies for only those with single ventricle repair (two in children following Glenn operation, seven in children following Fontan operation), and three included children regardless of heart defect. Children with single ventricle disease represent a distinct cohort with different underlying pathophysiology for pulmonary hypertension. Associated genetic anomalies, as well as altered fetal pulmonary blood flow, may contribute to altered pulmonary vascular development, and passive pulmonary flow following cavopulmonary anastomosis may contribute to underlying endothelial dysfunction in these patients. Reference Reddy, Siehr Handler, Wu, Rabinovitch and Wright86 Further study is required to define optimal timing of therapy initiation and discriminate between those children who would exhibit both haemodynamic and clinical improvement, and those in which therapy may actually increase morbidity and cost without a clinical benefit.
Fraisse et al attempted to compare differing doses of intravenous sildenafil in a multicentre trial, but the trial was prematurely closed, due to slow enrollment. Reference Fraisse, Butrous, Taylor, Oakes, Dilleen and Wessel41 The study planned for 228 children across 27 centres, but enrolled only 17 children across six centres. Their challenge exemplifies many of the difficulties with trials in this complex and high-risk population. Incompletely defined dosing ranges narrow the age of eligible children and infants, and disease heterogeneity limits the available study population. Furthermore, guardians may be hesitant to give consent for a placebo-controlled trial in a vulnerable population. Reference Zimmerman, Gonzalez, Swamy and Cohen-Wolkowiez87,Reference Torok, Li and Kannankeril88 Novel trial design may help overcome these barriers. Pragmatic trials that use real-world data and master protocols can support enrollment and reduce the need for trial-specific data collection and analysis. Reference Torok, Li and Kannankeril88,Reference Woodcock and LaVange89 Population pharmacokinetic modelling permits the study of drug disposition from sparse sampling methods and can be leveraged to simulate drug exposure from real-world dosing data to inform dosing strategies. Reference Hornik, Gonzalez and Dumond90 Indeed, recent analysis of milrinone highlighted the need for dose reduction in children with renal insufficiency. Reference Hornik, Yogev and Mourani69 Children with CHD undergoing surgery with cardiopulmonary bypass, especially those with underlying pulmonary hypertension, remain at high risk for post-operative morbidity and mortality, and further studies leveraging standardised endpoints and novel trial design are required to allow for evidence-based therapies and improved outcomes.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122002293
Acknowledgements
Erin Campbell, MS, provided editorial review and manuscript submission. Ms. Campbell did not receive compensation for her assistance, apart from her employment at the institution where this research was conducted. The authors would like to thank Duke Pediatric Research Scholars.
Conflicts of interest
Dr Foote reports no relevant disclosures. Dr Hornik reports no relevant disclosures. Dr Hill reports no relevant disclosures. Dr Rotta receives honoraria from BREAS US for participation in a scientific advisory board and royalties from Elsevier for editorial work. He has previously received honoraria from Vapotherm Inc for lecturing and consulting. Dr Kumar reports no relevant disclosures. Dr Thompson reports no relevant disclosures.





