Article contents
The Stability of Fe-Mg Chlorites in Hydrothermal Solutions: Ii. Thermodynamic Properties
Published online by Cambridge University Press: 01 January 2024
Abstract
The hydrothermal stabilities of a low-Fe clinochlore and a high-Mg chamosite, in the presence of kaolinite, were investigated recently at T ⩽ 200°C and Pv=PH2O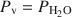 (Aja and Small, 1999; Aja and Dyar, 2002). Standard state thermodynamic properties (S2980
(Aja and Small, 1999; Aja and Dyar, 2002). Standard state thermodynamic properties (S2980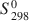 , ΔfH1,2980
, ΔfH1,2980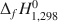 and ΔfG1,2980
and ΔfG1,2980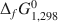 ) have been obtained for the two chlorites whose structural formulae are (Al2.33Fe1.002+Fe0.143+Ca0.02Mn0.01Ni0.02Cr0.01Mg8.40α0.07
) have been obtained for the two chlorites whose structural formulae are (Al2.33Fe1.002+Fe0.143+Ca0.02Mn0.01Ni0.02Cr0.01Mg8.40α0.07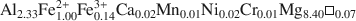 ) (Si5.66Al2.34)O20(OH)16 and (Fe0.603+Fe5.432+Mg2.30Al2.98Mn0.05Ca0.03Zn0.01α0.60
) (Si5.66Al2.34)O20(OH)16 and (Fe0.603+Fe5.432+Mg2.30Al2.98Mn0.05Ca0.03Zn0.01α0.60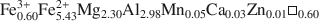 )(Si5.63Al2.37)O20(OH)16. For the low-Fe clinochlore, the respective thermochemical properties are 430 J mol−1 K−1, −8770.64±35.24 kJ mol−1, and −8120.54±32.63 kJ mol−1. ΔfH1,2980
)(Si5.63Al2.37)O20(OH)16. For the low-Fe clinochlore, the respective thermochemical properties are 430 J mol−1 K−1, −8770.64±35.24 kJ mol−1, and −8120.54±32.63 kJ mol−1. ΔfH1,2980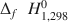 , ΔfG1,2980
, ΔfG1,2980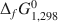 and S2980
and S2980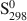 , similarly obtained for the Windsor chamosite are −7851.29±23.14 kJ mol−1, −7271.01±21.43 kJ mol−1 and 668±5 J mol−1K−1, respectively. Ideal site-mixing models of chlorite composition, along the chamosite-clinochlore binary, fail to model satisfactorily these chlorite-fluid equilibria only at lower temperatures (T <175°C). The magnitudes of the excess thermodynamic properties calculated for these chlorites, within the ternary clinochlore-daphnite-sudoite system, suggest significant deviations from ideality.
, similarly obtained for the Windsor chamosite are −7851.29±23.14 kJ mol−1, −7271.01±21.43 kJ mol−1 and 668±5 J mol−1K−1, respectively. Ideal site-mixing models of chlorite composition, along the chamosite-clinochlore binary, fail to model satisfactorily these chlorite-fluid equilibria only at lower temperatures (T <175°C). The magnitudes of the excess thermodynamic properties calculated for these chlorites, within the ternary clinochlore-daphnite-sudoite system, suggest significant deviations from ideality.
- Type
- Research Article
- Information
- Copyright
- Copyright © 2002, The Clay Minerals Society
References
- 9
- Cited by




