Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Serna, Carlos J.
White, Joe L.
and
Hem, Stanley L.
1978.
Structural Survey of Carbonate-Containing Antacids.
Journal of Pharmaceutical Sciences,
Vol. 67,
Issue. 3,
p.
324.
Mori, Kunio
Nakamura, Yoshiro
and
Kikuchi, Isao
1981.
Modification of poly(vinyl chloride). XLI. Effect of hydrotalcite on the stabilization of poly(vinyl chloride) by 6‐anilino‐1,3,5‐triazine‐2,4‐dithiol and zinc stearate.
Journal of Polymer Science: Polymer Letters Edition,
Vol. 19,
Issue. 12,
p.
623.
Serna, Carlos J.
Rendon, Jose L.
and
Iglesias, Juan E.
1982.
Crystal-Chemical Study of Layered [Al2Li(OH)6]+X−·nH2O.
Clays and Clay Minerals,
Vol. 30,
Issue. 3,
p.
180.
Kikkawa, S.
and
Koizumi, M.
1982.
Ferrocyanide anion bearing Mg, Al hydroxide.
Materials Research Bulletin,
Vol. 17,
Issue. 2,
p.
191.
Crovisier, J.L.
Thomassin, J.H.
Juteau, T.
Eberhart, J.P.
Touray, J.C.
and
Baillif, P.
1983.
Experimental seawater-basaltic glass interaction at 50°C: Study of early developed phases by electron microscopy and X-ray photoelectron spectrometry.
Geochimica et Cosmochimica Acta,
Vol. 47,
Issue. 3,
p.
377.
Miyata, Shigeo
1983.
Anion-Exchange Properties of Hydrotalcite-Like Compounds.
Clays and Clay Minerals,
Vol. 31,
Issue. 4,
p.
305.
Hernandez, M.José
Ulibarri, M.Angeles
Rendón, José Luis
and
Serna, Carlos J.
1984.
Thermal stability of Ni, Al double hydroxides with various interlayer anions.
Thermochimica Acta,
Vol. 81,
Issue. ,
p.
311.
Hernandez-Moreno, Maria J.
Ulibarri, María A.
Rendon, J. L.
and
Serna, Carlos J.
1985.
IR characteristics of hydrotalcite-like compounds.
Physics and Chemistry of Minerals,
Vol. 12,
Issue. 1,
p.
34.
Hernandez, M.J.
Ulibarri, M.A.
Cornejo, J.
Peña, M.J.
and
Serna, C.J.
1985.
Thermal stability of aluminium hydroxycarbonates with monovalent cations.
Thermochimica Acta,
Vol. 94,
Issue. 2,
p.
257.
Ulibarri, M.A.
Hernandez, M.J.
Cornejo, J.
and
Serna, C.J.
1986.
Textural properties of hydrotalcite-like compounds.
Materials Chemistry and Physics,
Vol. 14,
Issue. 6,
p.
569.
Sato, Tsugio
Kato, Kazuhiro
Endo, Tadashi
and
Shimada, Masahiko
1986.
Preparation and chemical properties of magnesium aluminium oxide solid solutions.
Reactivity of Solids,
Vol. 2,
Issue. 3,
p.
253.
Hernández, M. J.
Ulibarri, M. A.
and
Cornejo, J.
1986.
Kinetic study of the thermal dehydration of layered [Ni2Al(OH)6]2SO4.nH 2O.
Journal of Thermal Analysis,
Vol. 31,
Issue. 3,
p.
633.
Ulibarri, M.A.
Hernandez, M.J.
and
Cornejo, J.
1986.
Effect of heating on textural evolution on hydrotalcite-like compound [Ni2Al(OH)6]2SO4 · nH2O.
Materials Chemistry and Physics,
Vol. 14,
Issue. 3,
p.
209.
Mascolo, G.
1986.
Thermal stability of lithium aluminium hydroxy salts.
Thermochimica Acta,
Vol. 102,
Issue. ,
p.
67.
Drits, V. A.
Sokolova, T. N.
Sokolova, G. V.
and
Cherkashin, V. I.
1987.
New Members of the Hydrotalcite-Manasseite Group.
Clays and Clay Minerals,
Vol. 35,
Issue. 6,
p.
401.
Ulibarri, M.A.
Hernandez, M.J.
and
Cornejo, J.
1987.
Changes in textural properties derived from the thermal decomposition of synthetic pyroaurite.
Thermochimica Acta,
Vol. 113,
Issue. ,
p.
79.
Sato, Tsugio
Tezuka, Mikako
Endo, Tadashi
and
Shimada, Masahiko
1987.
Kinetics of anion uptake by rock salt-type magnesium aluminium oxide solid solutions.
Reactivity of Solids,
Vol. 3,
Issue. 4,
p.
287.
Suzuki, Eiichi
and
Ono, Yoshio
1988.
Aldol Condensation Reaction between Formaldehyde and Acetone over Heat-Treated Synthetic Hydrotalcite and Hydrotalcite-Like Compounds.
Bulletin of the Chemical Society of Japan,
Vol. 61,
Issue. 3,
p.
1008.
Trifirò, F.
Vaccari, A.
and
Piero, G.Del
1988.
Characterization of Porous Solids, Proceedings of the IUPAC Symposium (COPS I), Bad Soden a. Ts..
Vol. 39,
Issue. ,
p.
571.
Sugahara, Yoshiyuki
Yokoyama, Norimasa
Kuroda, Kazuyuki
and
Kato, Chuzo
1988.
AlN formation from a hydrotalcite-polyacrylonitrile intercalation compound by carbothermal reduction.
Ceramics International,
Vol. 14,
Issue. 3,
p.
163.
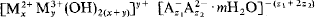 where M2+ and M3+ denote di- and trivalent cations, A− and A2− denote mono- and divalent anions, respectively, and y = z1 + 2z2; z1 ≫ z2.
where M2+ and M3+ denote di- and trivalent cations, A− and A2− denote mono- and divalent anions, respectively, and y = z1 + 2z2; z1 ≫ z2.



