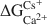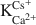Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by Crossref.
Sikalidis, C.A.
Misaelides, P.
and
Alexiades, C.A.
1988.
Caesium selectivity and fixation by vermiculite in the presence of various competing cations.
Environmental Pollution,
Vol. 52,
Issue. 1,
p.
67.
Singer, Arieh
1988.
Illite in aridic soils, desert dusts and desert loess.
Sedimentary Geology,
Vol. 59,
Issue. 3-4,
p.
251.
KOZAI, Naofumi
OHNUKI, Toshihiko
and
MURAOKA, Susumu
1993.
Sorption Characteristics of Neptunium by Sodium-Smectite.
Journal of Nuclear Science and Technology,
Vol. 30,
Issue. 11,
p.
1153.
Cornell, R. M.
1993.
Adsorption of cesium on minerals: A review.
Journal of Radioanalytical and Nuclear Chemistry Articles,
Vol. 171,
Issue. 2,
p.
483.
Lagaly, G.
1993.
Tonminerale und Tone.
p.
89.
Maes, E.
Iserentant, A.
Herbauts, J.
and
Delvaux, B.
1999.
Influence of the nature of clay minerals on the fixation of radiocaesium traces in an acid brown earth–podzol weathering sequence.
European Journal of Soil Science,
Vol. 50,
Issue. 1,
p.
117.
Delvaux, Bruno
Kruyts, Nathalie
Maes, Emmanuel
and
Smolders, Erik
2000.
Trace Elements in the Rhizosphere.
Gillot, F.
Righi, D.
and
Räisänen, M. L.
2001.
Layer-charge evaluation of expandable clays from a chronosequence of podzols in Finland using an alkylammonium method.
Clay Minerals,
Vol. 36,
Issue. 4,
p.
571.
Vandenhove, H.
Smolders, E.
and
Cremers, A.
2003.
Potassium bentonites reduce radiocaesium availability to plants.
European Journal of Soil Science,
Vol. 54,
Issue. 1,
p.
91.
Joussein, Emmanuel
Kruyts, Nathalie
Righi, Dominique
Petit, Sabine
and
Delvaux, Bruno
2004.
Specific Retention of Radiocesium in Volcanic Ash Soils Devoid of Micaceous Clay Minerals.
Soil Science Society of America Journal,
Vol. 68,
Issue. 1,
p.
313.
Degryse, F.
Smolders, E.
and
Cremers, A.
2004.
Enhanced sorption and fixation of radiocaesium in soils amended with K‐bentonites, submitted to wetting–drying cycles.
European Journal of Soil Science,
Vol. 55,
Issue. 3,
p.
513.
Bergaoui, L.
Lambert, J.F.
and
Prost, R.
2005.
Cesium adsorption on soil clay: macroscopic and spectroscopic measurements.
Applied Clay Science,
Vol. 29,
Issue. 1,
p.
23.
Vandenhove, H.
Cremers, A.
Smolders, E.
and
Van Hees, M.
2005.
Effect of K and bentonite additions on Cs-transfer to ryegrass.
Journal of Environmental Radioactivity,
Vol. 81,
Issue. 2-3,
p.
233.
Bergaya, F.
Lagaly, G.
and
Vayer, M.
2006.
Handbook of Clay Science.
Vol. 1,
Issue. ,
p.
979.
Burzo, E.
2007.
Phyllosilicates.
p.
366.
Roig, M.
Vidal, M.
Rauret, G.
and
Rigol, A.
2007.
Prediction of Radionuclide Aging in Soils from the Chernobyl and Mediterranean Areas.
Journal of Environmental Quality,
Vol. 36,
Issue. 4,
p.
943.
Nakao, Atsushi
Thiry, Yves
Funakawa, Shinya
and
Kosaki, Takashi
2008.
Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand.
Soil Science and Plant Nutrition,
Vol. 54,
Issue. 4,
p.
479.
Christidis, George E.
2010.
Advances in the characterization of industrial minerals.
p.
341.
Baker, Leslie L.
Strawn, Daniel G.
and
Smith, Robert W.
2010.
Cation Exchange on Vadose Zone Research Park Subsurface Sediment, Idaho National Laboratory.
Vadose Zone Journal,
Vol. 9,
Issue. 2,
p.
476.
NAKAO, Atsushi
2012.
Cesium Adsorption and Fixation on Soils.
TRENDS IN THE SCIENCES,
Vol. 17,
Issue. 10,
p.
10_40.