In Argentina, as in other countries, the number of reported pertussis cases has been increasing for over a decade [Reference Hozbor1]. Heightened public and provider awareness, improved diagnostics and surveillance, waning immunity, and bacterial evolution may explain the changing epidemiology of pertussis [Reference Hozbor1–Reference Tatti4]. An additional explanation may be an increase in the circulation of other Bordetella spp. that produce similar syndromes. To detect these different species, several PCR methodologies with different target sequences were designed. Some of these sequences are included in Supplementary Table S1 (available online). Although the IS481 PCR assay is very sensitive, it lacks specificity, as the B. holmesii and B. bronchiseptica genomes contain copies of IS481 [Reference Tatti4, Reference Njamkepo5]. Countries employing only the IS481 PCR assay for pertussis diagnosis lack the ability to distinguish B. pertussis from other Bordetella spp. Thus, a proportion of cases clinically and laboratory diagnosed as pertussis may be caused by species other than B. pertussis, such as B. holmesii.
A multi-target real-time PCR assay in combination with a single-target real-time PCR assay for ptxS1 has been developed for detection of B. pertussis, B. parapertussis, and B. holmesii [Reference Tatti4]. In 2010, the Argentine national reference laboratories began using this assay through collaboration with the US Centers for Disease Control and Prevention and the Latin American Pertussis ProjectFootnote †.
Cases included in this study were identified through the Sistema Nacional de Vigilancia de la Salud (National Health Surveillance System) between 1 July and 31 December 2010. Immediate reporting of suspected pertussis cases is mandatory in Argentina. A standard epidemiological reporting form collects clinical symptoms, demographic characteristics, vaccination history, and laboratory results for all suspected pertussis cases. This information is generally obtained when the patient is clinically suspected of pertussis. A nasopharyngeal aspirate or Dacron® swab specimen should be obtained from all suspected pertussis patients and tested by PCR or culture to attempt to confirm the diagnosis. The Argentine national laboratory network consists of 18 provincial and two national reference laboratories. All specimens are tested with conventional PCR based on IS481, ptx promoter and IS1001, which can distinguish between B. pertussis and B. parapertussis [Reference van der Zee6]. Specimens that are inconclusive or that are IS481 positive and ptx promoter or IS1001 negative are then tested at the national reference laboratories by the multi-target real-time PCR assay, which can additionally detect B. holmesii using the hIS1001-like primer probe set [Reference Tatti4]. Specimens are also tested by culture using charcoal agar plates (Oxoid, France) or Bordet-Gengou agar, both supplemented with defibrinated sheep blood. Suspected Bordetella colonies are presumptively identified by their phenotypic characteristics and then tested biochemically and by agglutination with antiserum for B. pertussis and B. parapertussis (Murex Diagnostic, UK).
Symptoms and vaccination history of PCR-confirmed B. pertussis case-patients identified at the National Reference Laboratory at La Plata during 1 July–31 December 2010, were compared with the same data from confirmed B. holmesii case-patients for the same period. We used Epi InfoTM version 3.5.3 (CDC, USA) for all analyses.
During the study period, 1475 suspected case-patients were tested for pertussis at the National Reference Laboratory at La Plata, 343 (23·9%) of which were positive for B. pertussis and nine (0·6%) for B. holmesii. The age distribution of pertussis cases was: <1 year (290 cases, 85·3%), 1–10 years (39 cases, 11·5%), and ⩾11 years (11 cases, 3·2%). Three case-patients’ ages were unknown. B. holmesii infection was identified by real-time PCR in all nine patients, two of which were also confirmed by culture. Six case-patients lived in the province of Santa Fe, and one each lived in Chubut, Entre Rios, and Neuquén provinces, representing a geographically dispersed population. The cases were temporally spread across the study period. Seven (78%) of the case-patients were aged <6 months, one was aged 7 months, and one was aged 13 years. Five (56%) of the case-patients were female. Only one case-patient was reported to have contact with a previously suspected case of Bordetella infection.
For all nine B. holmesii case-patients, catarrhal symptoms and non-paroxysmal cough were the most frequent (Table 1). Paroxysmal cough and fever were each reported by 1/3 of case-patients. Post-tussive vomiting occurred in one case. No B. holmesii case-patients reported apnoea, cyanosis, or inspiratory ‘whoop’. Mean duration of cough was 7·2 days (s.d. = 11·8, data not shown). No death was reported.
Table 1. Comparison of case-patient symptoms, Argentina, July–December 2010
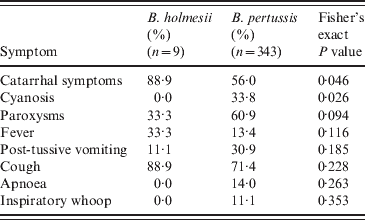
We observed that B. holmesii case-patients presented more frequent catarrhal symptoms (P = 0·046) and less frequent cyanosis (P = 0·026) and paroxysmal cough (P = 0·094). The two groups presented similar cough duration (μ = 8·4 for B. holmesii vs. 7·2 for B. pertussis; P = 0·8, data not shown). A comparison of vaccination status between the two groups did not produce a statistically significant difference (Fisher's exact P value = 0·547).
B. holmesii has been reported to cause respiratory symptoms, although it is associated primarily with bacteraemia in patients with underlying conditions [Reference Greig, Gunda and Kwan7, Reference Panagopoulos8]. Most data on B. holmesii infection, however, are from individual case reports. We are aware of two published reports of B. holmesii cases in patients suspected of pertussis that were identified through surveillance [Reference Miranda, Porte and Garcia9, Reference Yih10]. The majority of the B. holmesii case-patients identified in the Massachusetts study [Reference Yih10] was of adolescent (83%) or young-adult age (9%) with mild respiratory symptoms. Eleven case-patients in the Chilean report [Reference Miranda, Porte and Garcia9] positive for B. holmesii were spread across age groups, with three aged <12 months. In agreement with these last findings, we also detected B. holmesii in infants clinically diagnosed as pertussis. Although B. holmesii was detected in these patients, other possible pathogens were not tested for and therefore the data are suggestive, but not conclusive, that B holmesii was the responsible pathogen. Frequencies of catarrhal symptoms and cyanosis in these B. holmesii case-patients differed significantly from those of Argentinean patients with laboratory-confirmed B. pertussis. However, clinical presentations of B. holmesii cases were similar enough for clinicians to suspect pertussis and order a pertussis laboratory test.
We did not identify reports of secondary cases, and case-patients were from four dispersed provinces distributed across time, suggesting that these cases were not part of a localized outbreak. Increased awareness in the health system – due to the steady increase in cases of pertussis – and in laboratory capacity, such as incorporating multi-target real-time PCR methodology in 2010, increased the ability to identify this pathogen. Thus, B. holmesii may have been circulating unidentified in Argentina previously.
Regardless, detection of nine cases (eight aged <1 year) in a 6-month period is noteworthy in light of two retrospective studies' inability to detect B. holmesii in over 11000 Finnish and Dutch specimens collected between 1992 and 2003 or in 119 specimens positive for IS481 obtained from French patients aged <9 years between 2009 and 2010 [Reference Njamkepo5, Reference Antila11]. Our findings, which are similar to those of the Chilean report [Reference Miranda, Porte and Garcia9], may differ from those of the European countries because of a sustained increase in reported B. pertussis cases in Latin America over the past decade. Continued use of the multi-target, real-time PCR assay that can distinguish Bordetella spp. can assist in further monitoring the epidemiology and clinical picture of B. holmesii in Argentina.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S095026881200132X.
ACKNOWLEDGEMENTS
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica – ANCPyT and ANLIS – Dr. Carlos Malbran grants to D.F.H. and M.G. D.F.H. is a member of the Scientific Career of CICBA. D.B. and M.E.G. are in receipt of fellowships from Consejo Nacional de Investigaciones Científicas y Tecnológicas – CONICET.
DECLARATION OF INTEREST
None.



