INTRODUCTION
Tularemia is a multi-host, contagious, vector-borne zoonosis caused by Francisella tularensis, a bacterium with a broad host range, which includes invertebrates, mammals, and birds [Reference Olsufjev and Pavlovsky1, Reference Friend2]. There are four subspecies: F. tularensis subsp. tularensis (type A), F. tularensis subsp. holarctica (type B), F. tularensis subsp. mediasiatica and F. tularensis subsp. novicida. Types A and B are considered important for causing disease in humans and animals. Type A is present almost exclusively in North America and type B is distributed all over the Northern hemisphere, but predominately in Asia and Europe [Reference Keim, Johansson and Wagner3]. Tularemia-like symptoms in humans have been reported since the late nineteenth century in Europe, and it is believed that the so called ‘lemming-fever’ was caused by F. tularensis [Reference Friend2, Reference Morner4]. However, in recent years, tularemia has been shown to have a much broader range of hosts and infection routes than originally recognized. It is a zoonosis of complex epidemiology, and knowledge of reservoir hosts for this pathogen is still incomplete [Reference Keim, Johansson and Wagner3, Reference Tarnvik, Priebe and Grunow5]. Tularemia can be transmitted to humans through multiple routes, including contact with animals or contaminated environments, or through arthropod vectors; clinical presentation varies depending on the route of infection. Various epidemiological cycles appear to exist, which depend on the local topography, hydrography and presence of appropriate animal and vector species; each of these may present infection routes for humans [Reference Keim, Johansson and Wagner3, Reference Morner4]. Tularemia is important in Europe for a number of reasons. In humans it causes potentially severe disease if left untreated. Tularemia often presents with non-specific symptoms, which may delay its diagnosis. Sporadic human cases are often missed, particularly in areas which are assumed to have a low incidence of the disease [Reference Splettstoesser6]; this may lead to inefficient or delayed treatment, which may result in more severe manifestations of the disease. Tularemia has a broad geographical distribution, with sporadic cases and/or outbreaks occurring in many European countries. In the last 20 years, its geographical range has expanded to, or been increasingly recognized in, new areas, and the known host range has expanded to include species not previously linked with tularemia, such as the red fox (Vulpes vulpes), the wild boar (Sus scrofa) and the raccoon dog (Nyctereutes procyonoides) [Reference Hoflechner-Poltl7–Reference Kuehn10]. Tularemia has also re-emerged in a number of locations, notably causing new human outbreaks in southern and central Europe in recent decades [Reference Diaz de Tuesta11–Reference Grunow13]. Tularemia is, therefore, considered to be a locally emerging and/or re-emerging infection in Europe. Studies of tularemia have focused on different host species in different parts of Europe. Studies of wildlife in central Europe have involved targeted (or active) surveillance conducted in hunted hares, foxes, wild boar and trapped small rodents in endemic areas; these types of studies are scarce in Northern Europe. In the rest of Europe, studies are mainly based on passive surveillance of animals found dead or diseased, and these are primarily hares. Arthropod vectors play a role in the epidemiology of tularemia. Much of the research on the identification of possible arthropod vectors of tularemia has focused on ixodid ticks and on mosquitoes, but few studies have investigated the prevalence of F. tularensis subsp. holarctica throughout natural populations of different potential arthropod vectors in Europe. European studies have described certain aspects of tularemia or its presentations in limited geographical regions, but to date there has been no comprehensive overview of existing information on this disease in Europe, covering all its known host species and vectors. The aim of this study is to review the published and reported information and to provide an update on the geographical distribution of F. tularensis in humans, wildlife, domestic animals and vector species, and to identify potential public health hazards and to describe the current state of the epidemiology of tularemia in Europe. However, the type of reporting differs, and the investigations performed are of variable design, therefore the data compiled may not reflect the true situation of tularemia in Europe.
METHODS
Study setting
This review includes data from 38 countries: Member states (MS) of the European Union (EU), EU candidate and potential candidate countries and the four European Free Trade Association (EFTA) countries, hereafter called ‘the countries’. The review includes reported data and publications from 1992 to 2012 on human, wild and domestic animal cases of tularemia, and pathogen presence detected in arthropod vectors.
Human data
In the EU, tularemia in humans is reportable based on the Commission decision of 19 March 2002, which established case definitions for reporting communicable diseases to the Community Network under Decision No. 2119/98/EC of the European Parliament and of the Council, consolidated version 20120927 [14]. For the current review, the database of the European Centre for Disease Prevention and Control (ECDC) [15], created in 2005 was utilized. This database was queried for data on human cases of tularemia reported by EU MS, Norway, Liechtenstein and Iceland between 2006 and 2012. Complementary data were also extracted from the World Health Organization Centralized Information System for Infectious Diseases (WHO-CISID) database [16] for 1992–2012, which yielded yearly case numbers for 1992–2005 for the above-mentioned countries, and for the whole study period for Albania, Bosnia & Herzegovina, Croatia, Montenegro, Serbia, the FYR of Macedonia, Switzerland and Turkey. Case data by county/region and by year were also extracted from several national Public Health Institute websites. Population data produced by Eurostat [17] were used for incidence calculations. A literature review was conducted in order to find additional case data and important information on surveillance system, type of clinical presentation, and source of infection and exposure, in order to assess the disease risk in humans. The literature from January 1992 to December 2012 was searched using Pubmed, EMBASE, the Cochrane Library, OpenGrey and the DART Europe e-Theses Portal. The following search strategy was utilized (free text terms/medical subject headings and title/abstract): tularemia incidence, prevalence or outbreaks in humans, with the study setting ‘the countries’. Information on type of study, event description, frequency of clinical forms, routes and sources of infection, exposures and/or identified risk factors was extracted (Table 1). Thus, all countries reporting to the ECDC database have a comprehensive surveillance system for humans, but while reporting has been compulsory in most countries since 2003, it is still voluntary in Belgium and the UK. In Switzerland and Turkey, reporting of cases has been compulsory at the national level since 2004 [Reference Leblebicioglu18, Reference Wicki19]. The disease is not notifiable in Denmark, The Netherlands, Portugal and Liechtenstein. No information was available for Albania, Bosnia & Herzegovina, Croatia, Montenegro, Serbia and the FYR of Macedonia.
Table 1. Clinical presentations, routes of infection, type of exposure and risk factors of human tularemia in Europe
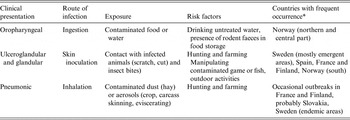
* In Germany and Czech Republic, all three types seem to have occurred in different years and regions.
Wild and domestic animal data
In animals, the disease is not reportable at the EU level (Council Directive 82/894/EEC on the notification of animal diseases within the Community amended and consolidated version 20130101 [20]), but is reportable at the international level (World Organization for Animal Health, OIE) according to the OIE listed diseases 2013 [21]. For the current review, information about tularemia and F. tularensis infection in wild and domestic animals was compiled from OIE databases and from published articles. Because the OIE databases only included data from 1996 onwards, data for the period from 1992 to 1995 was only available from published articles. The OIE databases used for this review were Handistatus II (1996–2004) [22], and the World Animal Health Information Database (WAHID, 2006–2012) [23]. However, it is important to note that notification (an official declaration to the OIE) is only mandatory when a new disease event occurs in a country; when a disease is endemic in a country, there is no longer any obligation to declare it. Thus, the information in OIE databases on the incidence of tularemia may not be an accurate reflection of the true extent of this disease. Further, this means that the information available differs among countries in the level of detail of the geographical location of outbreaks, and indeed, in whether all of the cases or outbreaks which occurred have even been reported. In this review the data are shown only as notification or no notification of tularemia at the country level. The literature from January 1992 to December 2012 was searched using the following databases: Web of Science, Cab Abstracts, Pubmed, Scopus and ProQuest. The search strategy was: tularemia cases, prevalence or outbreaks in wildlife or domestic animals, and the study setting ‘the countries’. Information on type of investigations conducted (active and passive surveillance, outbreak descriptions and reports of individual cases), disease status in wild and domestic animal species, estimated prevalence, source of infection, route of shedding and diagnostic methods used was extracted.
Vector data
For vectors there are no official databases or obligations to report. Databases and information are solely extracted from studies investigating different types of arthropod species. For the purpose of this review, a vector is any arthropod which can introduce F. tularensis into a susceptible host. A literature search of the databases Web of Knowledge, Science Direct and Google Scholar was conducted using the following search strategy: tularemia cases, incidence, prevalence or outbreaks in vectors or arthropods, and the study setting ‘the countries’. Reference lists of all reviewed articles were also assessed. Data extraction on prevalence rates of F. tularensis in European vectors was restricted to publications from January 1992 to December 2012. Data extracted included arthropod species, country, number of arthropods sampled, number of arthropods for which the test gave a positive response, prevalence of F. tularensis in arthropods studied, and the diagnostic method used. All studies regarding arthropod vectors were of the type active surveillance.
Mapping of data
Maps were compiled to compare the reported distribution of F. tularensis in Europe in humans, wildlife, domestic animals, and arthropod vectors, and the different types of F. tularensis surveillance in humans across Europe. Due to restrictions in the resolution of the available data, all information is shown at the country level. Information on the reported presence of F. tularensis in humans was obtained from the ECDC and the WHO-CISID databases. Maps of the reported presence of F. tularensis recorded in wildlife and domestic animals were prepared based on data obtained from OIE databases and from the literature search. Maps of the recorded presence of F. tularensis in arthropod vectors were based on the information obtained from the literature. Maps of the type of human surveillance which have been conducted at the national level were based on the information recorded by MS in the ECDC database and in the literature for Switzerland and Turkey. They concern coverage of surveillance (comprehensive or sentinel), notification type (compulsory or voluntary reporting), data sources (laboratory, hospital, general practitioner, health services, others) and case definition reference (for details see online Supplementary material). Country base layers were downloaded from Global Administrative Areas [24], and maps were created in ArcGIS.
RESULTS AND DISCUSSION
Tularemia in humans
During the twentieth century, according to Tärnvik [Reference Tarnvik, Priebe and Grunow5], there were large outbreaks of several hundred tularemia cases. These were associated with wartime conditions in various European countries during and after World War II (Austria, France, Hungary, Bulgaria, Germany), and with farming activities in Finland during the 1980s and 1990s. Four tularemia epidemics were reported from three different regions of Turkey between 1936 and 1953 [Reference Akalin, Helvaci and Gedikoglu25]. After a long interval, a new tularemia epidemic was reported from the area around Bursa in the northwestern part of Turkey in 1988, followed by small epidemics in various northwestern Turkish regions over a 10-year period. In recent years, European country reports of tularemia cases to international databases has largely improved the overall knowledge of the extent of the disease; in 1992 only 12 countries reported tularemia to the WHO, while in 2010, 31 countries reported to the ECDC/WHO. However, public awareness of the disease, and the surveillance systems used to detect the disease are not identical in the different European countries. Furthermore, the regularity and level of detail of reports submitted to international databases varies among the countries. Therefore, the current information on tularemia which is present in the international databases should be interpreted with caution.
Clinical presentations
Symptoms of tularemia are principally related to the site of entry of the bacteria, the virulence of the F. tularensis strain, and the immune status of the host. Tularemia has several clinical forms in humans, including ulceroglandular, glandular, pneumonic, oropharyngeal, oculoglandular and systemic (typhoidal, intestinal). In the ulceroglandular form, a local skin lesion is often considered the route of entrance (scratch, cut, insect bite) and the disease progresses to the swelling of the regional lymph node, which may ulcerate and suppurate. The glandular form of this disease is similar to the ulcerglandular form, but no primary skin lesion is detected in these cases. The pneumonic form results from inhalation of F. tularensis, and affects one or both lungs. The oropharyngeal form of tularemia is linked to ingestion of contaminated food or water. Other clinical presentations of tularemia, such as septic or typhoidal, are infrequent in Europe [Reference Tarnvik26].
Case numbers, incidence and outbreaks
Overall, 18 343 human cases of tularemia were reported to the WHO-CISID or ECDC databases between 1992 and 2012. Sweden reported 25% of all reported cases, Finland 22%, Turkey 13% and the Czech Republic and Hungary around 9% each.
Highest overall case numbers in Europe for the study period were observed in 2000, 2003 and 2010 with 1657, 1865 and 2458 cases respectively (Fig. 1). However, the number of reporting countries has increased over time, and the reported case numbers represented 19, 27 and 31 reporting countries, respectively. When analysed by country, high case numbers were reported by Finland and Sweden in 2000 (917 and 464 cases, respectively) and 2003 (823 and 798 cases, respectively), by Sweden in 2010 (484 cases), Norway in 2011 (178 cases), Spain in 1997 and 2007 (585 and 493, cases respectively), and Turkey in 2010 (1531 cases). The average incidence rate in the study area for 2006–2012, i.e. number of cases per 100 000 inhabitants, was 0·04; however, notably higher average incidence rates were seen in some countries. The highest average incidence rates were observed in Finland, followed by Sweden (4·84 and 3·78, respectively); relatively high average rates were also observed in Norway and Slovakia (1·16 and 0·93, respectively). When the data was examined by year, it was determined that Turkey experienced a high incidence rate in 2010 (2·11), Hungary experienced relatively high rates in 2006 (1·38) and 2010 (1·26), and Spain also had a high incidence rate in 2007 (1·11). Large variations of incidence rates occurred between years for each country, but also within each country, and the latter can be visualized at higher geographical resolutions. For example, very high incidence rates (above 82/100 000 inhabitants) were observed in Norrbotten (northern Sweden) in 2012 and in Finnmark (northern Norway) in 2011.

Fig. 1. Number of cases of tularemia by country and by year reported to the European Centre for Disease Prevention and Control (ECDC) and WHO databases between 1992 and 2012. Each country is represented by a different colour. Data for this figure come from Austria, Bosnia & Herzegovina, Bulgaria, Croatia, Czech Republic, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Lithuania, Norway, Poland, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland and Turkey, but not all countries reported for the entire period.
Additional epidemiological data compiled from the literature indicated that between 1992 and 1998 in Sweden, about 80% of the cases occurred in the northern part of the country, while in 1999 and particularly in 2000, a large proportion of cases also occurred in the middle and southern regions of the country [Reference Ekdahl and Twisselmann27]. During the period from 1995 to 2001, cases of tularemia in Finland were reported predominantly in the southern and western parts of the country (National Public Health Institute of Finland), while the northern and western parts of Finland have reported outbreaks since 2000 [Reference Kuusi, Klemets and Nuorti28]. The whole country of France seems to be affected, but some areas more heavily than others, a pattern which was similarly seen in the cases observed in hares [Reference Mailles29]. Similarly, more cases occurred in the western and southern parts of Germany, with an apparent increase in the number of cases after 2004 [Reference Splettstoesser and Seibold30]. In Norway, only the southern part of the country seems to have remained unaffected. Two war-related outbreaks occurred in Kosovo in 1999 and 2001 [Reference Grunow13], (WHO 2000). As indicated in Reintjes et al. [Reference Reintjes31], an outbreak of ulceroglandular tularemia, suspected to be associated with infected hares, was also reported in central and western Bosnia in 1995, in the aftermath of warfare. Serbia was also repeatedly affected in the early 2000s, in the region bordering Bulgaria. In Spain, in Castilla y Leon, two outbreak periods occurred at a 10-year interval (1997 and 2007); one isolated event happened in 1998 in Castilla la Mancha [Reference Diaz de Tuesta11]. In Turkey, outbreaks occurred in the northern half of the country in 2005 [Reference Akalin, Helvaci and Gedikoglu25], but during subsequent outbreaks in 2010–2012, the affected zone expanded to the more central parts of the country [Reference Balci12].
Outbreaks in humans are often sporadic and likely to be spatially and temporally variable, in addition to specific sites with potential for clusters of infection. Based on the reporting of human cases the major hotspots for tularemia at the European level are located in Scandinavia and Central Europe. According to data found in the literature, southeastern Europe (Slovakia, Serbia, Kosovo, Turkey) also show elevated incidences.
Trends
Data reporting has been inconsistent through the years and therefore trend analysis cannot be performed on all of ‘the countries’ at the same time. No clear trend was apparent in the data from 12 European countries which consistently reported cases between 1992 and 2012. The same was true for 23 countries which consistently reported between 2003 and 2010, and for EU MS, Norway and Iceland during the last 5 years (Fig. 2). However, trends were apparent in individual countries: Sweden, Norway, France, Germany and possibly Turkey (no recent data is available, but data from 2005 to 2010 was examined) showed a trend towards an increasing number of cases, while a trend of decreasing case numbers was apparent in Finland, Hungary, Bulgaria and Slovakia, and no trends appeared in data from Austria and the Czech Republic (Fig. 1, Table 2). An increase in case numbers was also reported for Switzerland [Reference Lyko and Chuard32].

Fig. 2. Number of cases of tularemia by month recorded in the European Centre for Disease Prevention and Control (ECDC) database, 2008 to 2012 (n = 4388), with a 12-month moving average. Data for this figure come from Austria, Belgium, Cyprus, the Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the UK.
Table 2. Summary of data* on tularemia in humans in Europe

* Data extracted from the European Centre for Disease Prevention and Control (ECDC) and WHO databases or the literature review (in italic) between 1992 and 2012, by country (only countries reporting cases in the last 6 years/with published articles during the study period are included).
† Number of cases per 100 000 population per year.
‡ Insufficient case numbers to calculate trends.
Tularemia in wild animals
Much of the basis of existing knowledge of tularemia in wild animals in Europe stems from research from the 1950s and 1960s, with the most extensive studies performed in the former Soviet Union. Olsufjev [Reference Olsufjev and Pavlovsky1] discussed tularemia in many species of wild animals including lagomorphs, murines, cricetines, carnivores, mustelids, artiodactyls and aves, providing information on ecology, epidemiology, bacteriology, and differences in host susceptibility. During the 1970s and 1980s, the mountain hare (Lepus timidus) in Sweden [Reference Morner33] and a variety of rodent species in several European countries were investigated. Tularemia in the European brown hare (Lepus europaeus) was studied in Hungary through serological surveys [Reference Gyuranecz34]. From the 1990s onwards, there was a broadening of the range of wildlife species examined for tularemia, and an increasing number of countries that conducted studies (Table 3). During the period 1992–2012, F. tularensis infection was most commonly investigated in the European brown hare, and in a variety of murine and cricetine species. Other species were also investigated, though less extensively; these included red fox, wild boar, mountain hare, lynx (Lynx lynx) and migratory shore birds. The investigations were conducted on dead animals obtained from hunters (hare, red fox, wild boar, lynx), through ad hoc sampling (hare, lynx, small rodents) and by trapping (murine and cricetine species, migratory shore birds). The methods used to detect either the bacterium F. tularensis itself, or antibodies to it, are shown in Table 3. The geographical distribution of F. tularensis at the country level is shown in Figure 3.

Fig. 3. Reported presence of tularemia in different host types across Europe 1992–2012, (a) presence in animals (reported in OIE database and literature), (b) presence in vectors (reported in literature), (c) presence in humans [reported in European Centre for Disease Prevention and Control (ECDC) and WHO-CISID databases for all countries except Switzerland and Turkey for which literature reports were used], and (d) type of human reporting at national level.
Table 3. Wild and domestic animal species investigated for infection with F. tularensis in Europe 1992–2012

FBFST, Fresh blood fast staining test; IFA, immunofluorescence test; IHC, immunohistochemistry; MAT, microagglutination test; PCR, polymerase chain reaction; SAT, slide agglutination test; TAT, tube agglutination test; WB, Western blot.
* In surveillance type investigations percentage, or range of percentage, are presented. For other study types occurrence is shown.
† Type of study: S, active or passive surveillance; O, outbreak description; I, individual cases. If an outbreak in humans or wildlife was the reason for conducting the study, this is shown as a subscript to S, O or I (h, outbreak in humans; w, outbreak in wildlife).
‡ Samples pooled from several individuals.
Most of the studies only diagnose F. tularensis to species, some also to subspecies holarctica. This is not detailed in the table.
Pathological presentations
According to previous publications, when infected with F. tularensis, the European brown hare develops chronic lesions [Reference Gyuranecz35], while the mountain hare typically develops acute fatal septicaemia [Reference Morner33, Reference Vikoren, Handeland and Djonne36–Reference Josefsen38]. However, studies from the last two decades indicate that the European brown hare can also develop acute disease [Reference Gyuranecz35, Reference Decors39]. In acute disease, necroses are distributed in multiple organs [Reference Morner33, Reference Decors39], while in chronic cases granulomatous inflammation can be seen in a more restricted number of organs, e.g. the kidney and lungs [Reference Gyuranecz35]. For other wildlife species, descriptions of the pathology and clinical presentations in naturally infected animals are still notably absent from the literature.
Serological investigations 1992–2012
Most of the serological investigations were conducted on samples from apparently healthy hunted or trapped animals. Results are presented in Table 3. Among the studied species, antibodies have been detected in European brown hare, red fox and wild boar. In Hungary, hares have been serologically surveyed yearly since 1984. For the period 1992–2010, the overall serological prevalence in 140 935 hares tested was 6·6% [Reference Gyuranecz34]. Studies finding serologically positive animals have also been published from Austria, the Czech Republic and Germany. In these studies, the prevalence in European brown hare, red fox and wild boar varied between 1·4% and 10·8% (Table 3). Serological investigations conducted during this time period in German and Hungarian small rodents, and in Swedish lynx did not detect any antibodies [Reference Gyuranecz40–Reference Ryser-Degiorgis42]. However, studies before 1992 did detect antibodies in Norwegian lemmings [Reference Berdal43] and Swedish beavers [Reference Morner, Sandstrom and Mattsson44, Reference Morner and Sandstedt45].
Detection of the bacterium F. tularensis 1992–2012
Table 3 summarizes results from investigations on the presence of the F. tularensis bacterium either in active surveillance studies or for diagnostic purposes, in single animals or small groups of animals. Many of the studies only diagnosed F. tularensis to species level, while others also determined the subspecies, which was holarctica in all reported studies. Apparently healthy hunted European brown hares were tested in Germany, with a detection rate for the bacterium varying between 0·3% and 2·9% [Reference Hauri46, Reference Runge47]. F. tularensis was detected in hares found dead in Germany [Reference Runge47], France [Reference Lamarque, Barrat and Moutou48] and Switzerland [Reference Haerer49]. In Germany, Runge et al. [Reference Runge47] also reported that 2·4% of wild European rabbits (Oryctolagus cuniculus) found dead tested positive for F. tularensis. Small rodents were investigated, primarily by trapping, in Austria, Bulgaria, Germany, Hungary, Kosovo, Slovakia and Sweden, and the detection rates varied greatly, from 0·7% up to 20·8%. Positive individuals were found among yellow-necked mice, wood mice, house mice, striped field mice, bank voles, common voles, water voles, field voles and black rats [Reference Kaysser41, Reference Broman50]. In one study in Austria, F. tularensis was detected in 1·3% of the submandibular lymph nodes of apparently healthy hunted red foxes [Reference Hofer9]. In Portugal, 212 migratory shore birds of varying species were examined for bacteria in their blood, which resulted in the identification of F. tularensis in one little stint (Calidris minuta) [Reference de Carvalho51].
Tularemia in domestic animals
Outbreaks of tularemia in domestic animals have been reported to the OIE by 11 European countries (Fig. 3). Of the eight countries that have stated the affected species, seven reported tularemia in farmed hares/rabbits and only one country (Bulgaria) has reported tularemia in other species, specifically in cattle, sheep and dogs. Only a few papers or case reports on tularemia in domestic animals were found in the literature, and these only presented information on seropositivity for tularemia in domestic animals in European countries (Table 3). These studies often resulted from investigations of possible sources of infection causing outbreaks in humans, and presented no information on clinical disease in domestic animals.
Tularemia in vectors
Arthropods have long been associated with the transmission of Francisella tularensis, with many of the early studies focusing on deer flies [Reference Jellison52] as potential vectors. The range of arthropod vectors connected with tularemia has expanded to include ticks [Reference Wicki19, Reference Gyuranecz40, Reference Gurycova53–Reference Gehringer60], mosquitoes [Reference Svensson56, Reference Lundstrom61], horse flies [Reference Svensson56], fleas [Reference Gurycova62] and gamasid mites [Reference Keim, Johansson and Wagner3]. In Europe, much of the research on the role of arthropod vectors in the transmission of F. tularensis subsp. holarctica has focused on ixodid ticks (hard ticks) in central Europe and on mosquitoes in northern Europe (e.g. Sweden and Finland) (Table 4). These geographical differences in research effort and the role of these two vector species in the transmission of tularemia are largely based on the regional abundance of the two vector groups, with mosquitoes being in far greater abundance in northern Europe, while ticks are relatively rare [Reference Keim, Johansson and Wagner3]. However, there have been surprisingly few studies investigating the prevalence of F. tularensis subsp. holarctica in natural populations of vectors in Europe, and they cover a relatively narrow range of countries (Table 4).
Table 4. Studies estimating prevalence of F. tularensis in European arthropods 1992–2012

IWM, Inoculation on white mice; PCR, polymerase chain reaction.
* All studies are of active surveillance type.
† Samples pooled from several individuals.
Most of the studies only diagnose F. tularensis to species, some also to subspecies holarctica. This is not detailed in the table.
Ticks
There is strong evidence that the tick is a biological vector of F. tularensis. The bacteria has been found in the midgut and salivary glands [Reference Reif63] of ticks and has also been shown to replicate within the tick [Reference Reif63]. Ticks have been shown to be capable of transmitting the infection during a blood meal [Reference Reif63, Reference Petrov64] and mechanically via interrupted feeding [Reference Petrov64]. Furthermore, transstadial transmission of F. tularensis has been demonstrated experimentally in ticks [Reference Petrov64, Reference Petersen, Mead and Schriefer65] and field studies have isolated F. tularensis from a number of adult male Dermacentor reticulatus ticks [Reference Hubalek and Halouzka54]. Given that adult male D. reticulates ticks do not take blood meals, this suggests that transstadial transmission may have occurred. Transovarial transmission of F. tularensis has however not been shown to occur experimentally in ticks, although there have been reports of infected unfed larvae in the field [Reference Petersen, Mead and Schriefer65].
The prevalence rates detected in ticks have been mostly between 0% and 3% (Table 4). However, it should be noted that some studies estimated prevalence based on the testing of individual ticks, while others estimated prevalence by testing a pooled set of ticks. The latter type of study may not accurately reflect the prevalence of the bacterium in the tick population. This is highlighted in two studies which reported unusually high prevalence rates of 16% [Reference Hubalek and Halouzka54] and 8% [Reference Gehringer60] which were based on pools of 8–13 ticks, and therefore may not be an accurate estimation of prevalence in the population of ticks tested. Furthermore, ticks have also been found to harbour Francisella-like endosymbionts (FLEs). FLEs are non-pathogenic bacteria which are closely related to F. tularensis [Reference de Carvalho58]. These FLEs pose a challenge for accurate identification of F. tularensis by PCR as the 16S rDNA gene sequence is highly conserved between these two species [Reference Michelet66]. Thus, studies that have not used tools capable of discriminating between F. tularensis and FLEs may overestimate the prevalence of F. tularensis in ticks.
Mosquitoes
In contrast to ticks, there is less information on the role of mosquitoes as biological vectors of F. tularensis.
Lundström and colleagues isolated F. tularensis subsp. holarctica from adult mosquitoes that were collected from the field as larvae and reared in the laboratory [Reference Lundstrom61]. This suggests that the larvae acquired F. tularensis in the field, and maintained the bacteria until they were tested as adults. Furthermore, mosquito larvae predate on protozoa that have been shown to harbour F. tularensis subsp. holarctica [Reference Abd67], suggesting that it is possible that mosquitoes may acquire the infection as larvae. Transstadial transmission was also confirmed in experimental studies by Thelaus et al. [Reference Thelaus68], where 25% of the adults of Aedes aegypti infected with F. tularensis subsp. holarctica as larvae maintained the bacteria. When uninfected mosquitoes fed on infected mice they were able to acquire the bacteria. In contrast, laboratory experiments by Triebenbach et al. [Reference Triebenbach69], infecting larvae from A. aegypti and Anopheles gambiae with F. tularensis subsp. novicida concluded that there was no evidence of transstadial transmission, that mosquitoes were unable to acquire the bacteria during a blood meal and that infected mosquitoes were not able to transmit the bacteria during a blood meal. Neither the study by Thelaus et al. or Triebenbach et al. could show transmission of the bacteria from infected mosquitoes to uninfected mammal hosts. They also agree that there is no evidence for active replication of the bacterium within the mosquitoes [Reference Thelaus68, Reference Triebenbach69].
There have been relatively few studies investigating the prevalence of F. tularensis in mosquitoes (Table 4). However, when prevalence of F. tularensis was estimated in mosquitoes, it was reported to be greater than that observed in ticks (a prevalence of 8–21%), although it should be noted that this was only based on one study [Reference Lundstrom61].
Linking humans, wildlife and arthropod vectors
Susceptibility to disease in different animal species
Sensitivity to infection, and thus the severity of the resulting disease, differs between animal species. In the WHO Guidelines on tularemia [Reference Tarnvik26], a host's potential sensitivity to F. tularensis spp. (including subspecies tularensis and holarctica) infection is classified as ‘very sensitive’, ‘medium sensitive’ or ‘resistant’, based on data from experimental infections. According to the WHO Guidelines [Reference Tarnvik26], several small rodent species are considered very sensitive to the pathogen, which can be exemplified by the experimental infection conducted on common hamster (Cricetus cricetus) leading to septicaemic disease and death [Reference Gyuranecz70]. Experimentally infected lagomorphs, have been considered to be very sensitive and develop acute disease [Reference Tarnvik26]. In Europe this has been shown in experimentally and naturally infected hares with F. tularensis subsp. holarctica [Reference Morner33, Reference Decors39, Reference Borg71]. However, studies of naturally infected European brown hares (L. europaeus) have also described chronic disease [Reference Gyuranecz35], which suggests that the disease in hares does not always have an acute and fatal presentation. Antibodies against tularemia have been detected in Eurasian beavers (Castor fiber) [Reference Morner and Sandstedt45], but unfortunately there are no studies published on the disease in naturally infected Eurasian beavers in Europe. Olsufjev (Olsufjev, 1970, cited in [Reference Tarnvik26]) suggests that some resistant genera are Canis, Felis, Meles, Nyctereutes, Vulpes and Mustela. There are, however, occasional descriptions of naturally infected and diseased domestic cats and dogs [Reference Friend2]. Birds have been experimentally infected, and most species seem to be able to become infected, but are relatively resistant to developing clinical disease [Reference Friend2]. Humans are considered to be highly susceptible to infection, resulting in a disease that ranges from mild to severe [Reference Friend2, Reference Tarnvik26]. It is important to take into account the differences in sensitivity between species in order to understand the epidemiological role of the various possible host species, and to design appropriate surveillance strategies. However, the information available on the susceptibility of European hosts to F. tularensis subsp. holarctica is limited and there are few descriptions of experimental infections and natural disease. A large part the information available (see above) is outdated and often contains no information on the subspecies of F. tularensis involved.
Geographical distribution of tularemia in Europe 1992–2012
Tularemia is widely distributed in humans, wildlife and arthropod vectors in Europe. Figure 3 maps the distribution of F. tularensis subsp. holarctica across Europe; it clearly has a broad distribution, with areas of reported presence spanning from the northernmost European countries to the southernmost ones, and from the east across to the west. It can be clearly seen from Figure 3 that tularemia is present in both humans and wildlife in the majority of European countries. However, while human cases have been reported from Estonia, Latvia, Lithuania, Slovenia, Croatia, Bosnia & Herzegovina, Serbia, Turkey and Romania, there have been no reported cases in wildlife. In Belgium there have been reports of tularemia in wildlife, but not in humans, either in the databases or the literature. In Denmark, the only wildlife case reported was in a European brown hare, and there was a single human case described in the literature [Reference Bystrom72]. Tularemia cases in domestic animals have been reported from Bulgaria, the Czech Republic, Germany, Finland, France, Italy, Norway, Poland, Slovakia, Spain, Sweden and Switzerland. Many of these were in domestic lagomorphs. F. tularensis subsp. holarctica was only reported in arthropod vectors in some of the countries where outbreaks in other hosts were also observed. One possible explanation for these differences is that the only source of information on F. tularensis in vectors is published articles, while international databases have further information on the pathogen in humans, wildlife and domestic animals. It is difficult to compare the prevalence of tularemia, both between species (humans, wildlife, domestic animals and vectors) and between countries. There have been differences in the levels of surveillance between countries, and in the level of reporting engaged in by each country, ranging from no reporting to compulsory reporting, which affects the number of cases in the databases. Notification of animal cases is mandatory at the international level. The purpose of official notification of tularemia cases is to enable the veterinary authorities of a tularemia-free country to prohibit importation or transit of live hares through their territory from countries considered infected with tularemia. As a result, some countries are reluctant to report cases, as they might suffer economic disadvantages. A further explanation for differences in levels of tularemia in the different species may be attributable to the different methods of surveillance utilized: Human cases are recorded through passive surveillance systems, while wildlife and domestic animal reports result from both passive and active, and vectors from active surveillance.
Seasonality of disease
The majority of human cases reported between 2007 and 2012 in EU countries, Norway and Iceland occurred from July to November, with a peak typically occurring in August or September. However, some cases were also reported in February and March.
Tularemia thus appears to have a clear seasonal pattern in humans in the EU, with most cases occurring in the summer and early autumn months (Fig. 4, see also trend graph in Fig. 2). However, when examined at the individual country level, the pattern of seasonality appeared highly variable. In Italy, cases were more often reported in spring; in Hungary, in summer; in Sweden, Finland and Spain, in summer/autumn; in Norway, in autumn/winter; in Germany, in winter; and in Slovakia, France and the Czech Republic, all year round with a slight peak in winter (see Table 2). Data was either missing or insufficient to assess seasonality in the other countries. According to the literature reviewed, outbreaks were reported: in spring/summer/autumn in Bulgaria, Finland, Slovakia and Sweden; in autumn/winter/spring in Kosovo and Turkey; and in all seasons in France, Germany, Norway and Spain (see Table 2). Thus, in some countries, outbreaks occurred in periods during which the Europe-wide average reported incidence was low.

Fig. 4. Number of cases of tularemia by month recorded in the European Centre for Disease Prevention and Control (ECDC) database (n = 5715), 2007 to 2012. Data for this figure has been reported by Austria, Belgium, Cyprus, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the UK.
In wildlife, previous studies have reported seasonal patterns of outbreaks in Northern Europe, which researchers have associated with the peak of the mosquito season [Reference Morner33]. From 1992 to 2012, a study was conducted on Norwegian mountain hares, which were found dead between May and November, and had died of tularemia [Reference Vikoren, Handeland and Djonne36–Reference Josefsen38]. These hares had septicaemic (acute) disease; as this form of tularemia results in death within a few days, it is likely they were infected shortly before death. Since the mosquito season spans from about June to September in Scandinavia, the timing for these infections would be consistent with the hypothesis that the mosquitoes played an important role in spreading the infection. Information from studies on European brown hares in Hungary, by contrast, have not been as informative with respect to the time of infection and identification of possible vectors. In Hungary, hares have been examined primarily during hunting season; in those infected with tularemia, the lesions were consistent with subacute to chronic disease, making it difficult to establish at what time point the hares contracted the infection [Reference Gyuranecz35]. Efforts have been made to detect F. tularensis in small rodents in endemic areas in Bulgaria, at the Austrian-Slovakian border, and in Hungary and Sweden (Table 3). Only two studies, both on the Austrian–Slovakian border, presented results by season [Reference Gurycova73, Reference Vyrostekova74]. The combined results showed a bacterial detection rate of 0% in January–June, 1·2–2·6% in June/July–September and 3·9–8·3% in October–December. It is not possible to determine if these results reflect a true seasonality, since the studies were small, and limited in both time and geographical location.
Reports of tularemia in domestic animals have been almost exclusively about farmed hares/rabbits [22, 23] and give no information on seasonality.
Human disease and risk factors
The most frequent clinical presentations of tularemia in humans in Europe, routes of infection, type of exposure, and risk factors are all summarized in Table 1. Oropharyngeal outbreaks were more frequent in autumn/winter/spring in most of the countries, associated with exposure to contaminated food or water, while ulceroglandular forms occurred mostly in summer or autumn, associated with exposure to infected vectors or hares (see also Table 2). The most frequently reported information on wildlife, associated with all forms of human outbreaks, was observations of increased numbers of rodents or dead hares, or contact with these (Table 2). Infection can indeed occur through direct contact, for example when hunters skin infected hares. Two outbreak investigations in Spain in 1998–1999 and 2007 mentioned handling of red crayfish as a risk factor for infection [Reference Anda75, Reference Allue76]. From the literature reviewed in the current study, the most frequently mentioned risks for humans in Europe to contract tularemia were from: consumption of contaminated water or food (mentioned 20 times), direct (skin) contact with infected animals – frequently wild ones (mentioned 19 times), vector bites from infected arthropods (mentioned 13 times), and exposure to infected environments via air (mentioned 10 times). Males in general and rural populations were found to be more often occupationally exposed (mentioned 11 times), urban populations to be affected through recreational outdoor activities, like hunting and fishing (mentioned 10 times). Contact with domestic animals has on occasion been implicated as a risk factor in connection with tularemia outbreaks in humans [Reference Leblebicioglu18, Reference Allue76–Reference Guercan79]. The few papers or case reports on seropositivity to F. tularensis in domestic animals in European countries (see Table 3) often resulted from investigations of potential risk factors for human outbreaks. Apart from those studies, evidence of the link between tularemia in domestic animals and humans in Europe was often circumstantial, and traced to reports of humans in whom infection was detected after they were scratched or bitten by cats [Reference Rodon80, Reference Yaqub, Bjørnholt and Enger81] or had been fleecing sheep [Reference Senol82]. The information regarding tularemia in zoo animals is scarce and describes mostly outbreaks or single cases in non-human primates. However, recently Kuehn et al. [Reference Kuehn10] performed serological investigations of 1122 German zoo animals representing 46 different species, most of them ungulates. Three (0·3%) tested positive for antibodies against tularemia. Contact between wild animals, e.g. small rodents, and zoo animals located in the same geographic area might enable transmission of tularemia between these animal groups. This study showed low seroprevalence and therefore the risk for humans to contract infection from zoo animals was likely low.
Sources of infection and routes of dissemination
Wild animals and arthropods appear to be important sources of infection with F. tularensis for humans and other animals. In theory, species that are moderately susceptible to tularemia, and maintain the infection for a prolonged time, may serve as reservoirs. In other hosts, bacterial amplification can occur, and they may serve as a source of infection to others [Reference Haydon83]. It has been shown that sensitivity to F. tularensis infection, and susceptibility to develop disease, varies between animal species. The sources of infection for animals have not been well investigated, but it is reasonable to hypothesize that they could be infected by routes similar to those for humans, i.e. through direct contact with secretions from infected animals, from ingestion of contaminated food or carcasses, via vectors, or from inhalation of the bacteria. Only a few studies have investigated the distribution of lesions in wildlife to determine in which organs lesions were present. Similarly, few studies have investigated possible routes of infection and shedding of F. tularensis in wildlife. In Hungary, hunted European brown hares with tularemia had subacute to chronic lesion in the lungs, pericardium, and kidneys [Reference Gyuranecz35]. Because lung involvement was a common finding, the authors postulated a respiratory route of infection. In terms of transmission of the disease, hares with chronic disease in the kidney could, in theory, shed bacteria in urine and thereby serve as a source of infection. This has not been shown in hares, but in experimental infection of meadow vole (Microtus pennsylvanicus) the animals developed bacteriuria [Reference Bell and Stewart84]. Olsufjev [Reference Olsufjev and Pavlovsky1] reported that the organs and blood of small rodents contained numerous bacteria, which could pose a risk for transmission to other animals either through shedding by the rodents during the acute phase of the disease, or as contaminated carcasses once the animals died. Most of the descriptions of transmission from small rodents to humans resulted from contact with dead infected animals, for example as reported through skinning of hamsters [Reference Munnich and Lakatos85]. There is very little information about their importance as live shedders or as reservoir hosts. Naturally infected small rodents have only been investigated for the presence of the agent, but not for lesions [Reference Reintjes31, Reference Gyuranecz40, Reference Kaysser41, Reference Broman50, Reference Gurycova73, Reference Christova and Gladnishka86, Reference Berdal87]. The fact that these small rodents were alive while harboring the bacterium might reflect that they acted as healthy carriers, had chronic disease, or had been recently infected and had not yet developed disease. Small rodents have also been implicated in the spread of tularemia through the contamination of food and water. In Norway, outbreaks of tularemia in humans have been attributed to drinking water from water-wells contaminated by dead lemmings or their excreta [Reference Larssen88]. Similarly, in Kosovo an outbreak of oropharyngeal tularemia in humans was attributed to contamination of food and water with rodent faeces [Reference Reintjes31]. From the studies conducted to date, it is not clear if small rodent species act as amplifiers of tularemia infection or might be reservoirs, or if their role differs in different areas, given that multiple factors are involved in the eco-epidemiology of tularemia. Red fox, wild boar and raccoon dog may contract infection (Table 3, [Reference Kuehn10]) when eating infected prey such as hares and small rodents and develop antibodies. However these species are not considered to be particularly susceptible to developing disease. Their potential role as asymptomatic carriers, shedding and/or spreading the infection in nature, has not been investigated [Reference Olsufjev and Pavlovsky1, Reference Hoflechner-Poltl7, Reference Hofer9, Reference Hubalek89, Reference Al90].
Eco-epidemiology of tularemia
The epidemiology of F. tularensis subsp. holarctica infection is complex and varies with ecosystem and geographical region. There are considerable gaps in the knowledge of the role of arthropod vectors, wildlife, and the environment in maintenance of the infection in natural habitats. Both a terrestrial and an aquatic ecological cycle of tularemia have been described, each involving their respective populations of mammal and arthropod vector hosts and their environments [Reference Gyuranecz34]. DNA from F. tularensis has also been found in water and sediment, suggesting that the bacteria persist silently in the environment [Reference Broman50]. Highly endemic areas or natural foci of tularemia have been known for several decades [Reference Olsufjev and Pavlovsky1, Reference Gurycova73]. In these areas, cases often occur every year, and in higher numbers, than in less heavily affected endemic areas [Reference Tarnvik, Sandstrom and Sjostedt91]. For example, areas of high endemicity have been identified in both Germany and Sweden [Reference Runge47, Reference Tarnvik, Sandstrom and Sjostedt91]. In Sweden, the theory of nidality/focality has been applied to the outbreaks of tularemia, where molecular typing of F. tularensis subsp. holarctica in human cases revealed highly localized sub-populations of F. tularensis of identical genotypes detected over multiple years [Reference Svensson56]. This suggests that the human cases of tularemia may have originated from distinct local sources of infection, and that overwintering of the disease may have occurred at these sites. In laboratory experiments F. tularensis subsp. holarctica has been shown to survive in water for several months [Reference Broman50], and given the strong association of F. tularensis subsp. holarctica with water, it is possible that mosquitoes play a role in transmission of tularemia from a water reservoir to humans. Although there is no experimental data to support this assertion, the timing of human outbreaks tend to be localized to summer and autumn, when mosquito numbers are at their greatest, and this suggests there may be a connection. Rydén and colleagues [Reference Ryden92] recently reported a significant correlation between mosquito abundance and human cases of tularemia. At present, transmission of F. tularensis from infected mosquitos has not been proven since it has not been shown experimentally [Reference Thelaus68, Reference Triebenbach69]. However, Thelaus et al. [Reference Thelaus68] suggest that the transmission from infected mosquitoes occurs at a very low frequency and therefore could not be detected in their experiment.
It has often been reported that ticks play a significant role in maintaining F. tularensis in nature and may be a reservoir species for tularemia [Reference Keim, Johansson and Wagner3, Reference Petersen, Mead and Schriefer65, Reference Akimana and Kwaik93]. There are aspects of tick biology which provide support for this possibility. Ticks are capable of transstadial transmission. Furthermore, the life-cycle of most ixodid ticks is completed over 2–3 years and they go through three life stages, each requiring a blood meal (larva, nymph, adult) [Reference Petersen, Mead and Schriefer65]. Studies on the long-term prevalence rates of F. tularensis in ticks provides further support for the theory that ticks may be a reservoir species for tularemia. In the USA, there has been evidence that F. tularensis subsp. tularensis persisted in tick populations at prevalence rates of 2–4% for 4 years in Martha's Vineyard [Reference Goethert and Telford94]. Similarly, in Europe, Gurycová et al. [Reference Gurycova53] found that F. tularensis subsp. holarctica persisted at prevalence rates of 0·2–1% in ticks in western Slovakia for 6 years in an area in which tularemia was endemic. It has been proposed that the long-term existence of F. tularensis in ticks is consistent with the theory of natural nidality/focality where infections are maintained in natural nodi or foci, and spillover to other hosts/environments occurs during periods of disruption, which may cause amplification [Reference Ryden92, Reference Goethert and Telford94, Reference Goethert, Saviet and Telford95]. However, the point at which these spillover events happen is unknown. There is conflicting evidence for the theory that ticks maintain the tularemia infection in nature, as a number of studies have found that infection with F. tularensis negatively affects the survival of ticks [Reference Petrov64, Reference Goethert and Telford94], which would impact the ticks' ability to maintain the infection in the population.
Surveillance of wildlife and humans
Surveillance data, based on an official declaration of cases in animals or in humans, can be used to map the presence of tularemia over time. This information can then be used to estimate the risk of exposure for various populations in different locations. However, because the current level of awareness of tularemia is poor, it is quite possible that the risk of exposure for humans or domestic animals has not been adequately assessed. In recent years, tularemia has been found in areas not previously known to be affected; for example, in a hare in Thuringia in Germany 2006 [Reference Muller8] and in humans in Kosovo during 1999–2000 [Reference Reintjes31]. Re-emergence has occurred in humans in several regions of Turkey after decades during which there had been no outbreaks of the disease [Reference Akalin, Helvaci and Gedikoglu25, Reference Gurcan96].There have been outbreaks of tularemia in humans in areas where the disease had previously been rare, both in Sweden in 2000 [Reference Eliasson77] and in Germany in 2004 [Reference Kaysser41]. In some locations, there was evidence that the peaks of infection coincided in humans and in wild animals (e.g. hares) [Reference Morner4, Reference Gyuranecz34]. This pattern was not, however, consistently seen during the study period from 1992–2012. This may be because this pattern of concurrent human and wildlife outbreaks only occurs in particular geographical locations, or it could be because detection of such patterns requires studies specifically aimed at simultaneously investigating tularemia in humans and wildlife. This latter type of study has not often been undertaken. Recent studies which used a more comprehensive approach to surveillance identified F. tularensis or antibodies to F. tularensis in species which had not previously been well-investigated, such as raccoon dog, wild boar and red fox [Reference Hoflechner-Poltl7, Reference Hofer9, Reference Kuehn10]. Thus, tularemia may be more widespread in the environment than has been generally recognized, which may be resulting in emerging and re-emerging disease in humans who are exposed to contaminated areas of the environment through high risk activities. Red foxes, wild boar and raccoon dogs are all wildlife species that can contract tularemia infection (usually by preying on other infected wildlife), but rarely develop disease. These species might therefore serve as useful indicators for the presence of tularemia in areas where small mammals are infected. Furthermore, they can be used for serological surveillance of trends in tularemia and for the detection of spread of the pathogen to new geographical areas [Reference Hoflechner-Poltl7, Reference Hofer9, Reference Kuehn10, Reference Hubalek89, Reference Al90]. Serology in hares has been used for wildlife surveillance of tularemia in Hungary [Reference Gyuranecz34], where results from regular testing of hunted hares for almost three decades has identified variation in prevalence of the pathogen over time in different parts of the country. Because hares often succumb to acute disease, early detection of outbreaks in this species can provide an early warning for regional health authorities. These authorities could then help people reduce their occupational or recreational exposure, by providing advice regarding precautions which might be taken to avoid sources of infection such as avoiding contact with hares and bites by arthropod vectors. This strategy was employed during an outbreak of tularemia in hares in France in 2011; health officials enhanced local surveillance efforts, and warnings were issued to local people at risk (e.g. hunters and people walking in the forest) and to physicians to aid in prevention and early detection of disease [Reference Decors39]. In Lower Saxony in Germany, systematic testing of hares which were hunted or found dead, revealed highly endemic areas of tularemia which were previously unknown [Reference Runge47]. Small rodent species are also useful for active surveillance, as several studies [Reference Broman50, Reference Gurycova73, Reference Vyrostekova74, Reference Christova and Gladnishka86] have demonstrated that they are potential carriers of the bacterium and may be reservoirs of infection [Reference Vaissaire97].
CONCLUSIONS
The results of this review and conclusions drawn must be interpreted with caution. The results in humans and animals are based on different types of reporting with variable level of detail and differ between countries, in some reporting of tularemia is compulsory and in others it is not. Moreover, the published articles are of different types, ranging from large surveillance studies to descriptions of single cases. Therefore, this review study has limitations and may not necessarily reflect the true situation of tularemia in Europe.
An increase in the breadth and depth of studies on tularemia, in combination with the development of new and better diagnostic methods, have increased the knowledge and detection of tularemia in humans, wildlife and arthropod vectors over the past 20 years, although many aspects of this disease still remain poorly understood. There is a lack of knowledge and understanding of the epidemiological role of animal hosts, mechanisms of maintenance in the different ecosystems, and routes of transmission.
Reporting of tularemia has increased in many European countries over the past 20 years. This may be attributable to the increase in the mandatory reporting of this disease but also to better awareness and diagnostic possibilities. Over the past 5 years, there has been no overall trend of infection in the EU, Norway and Iceland when taken as a whole, but there is considerable variation at the individual country level.
Tularemia is widely distributed throughout most of Europe and has repeatedly shown signs of local emergence and re-emergence in humans and wildlife. The disease shows clear seasonality in humans but this pattern has not been definitively demonstrated in wildlife.
Different geographical areas have different ecosystems, which influence the epidemiology and presentation of the disease. Such factors include temperature and humidity, the presence of different kinds of arthropod vectors, and the variety of small rodent species and other wildlife species present. This makes it difficult to make direct comparisons across all of Europe.
Increased active surveillance of tularemia, simultaneously investigating humans, wildlife, domestic animals and vectors is recommended. This will contribute to the understanding of the role of the different animal species and vectors in the development, transmission, and maintenance of the disease. Development of simple, field-based diagnostic methods, applicable to various animal species, would facilitate field investigations.
Better communication among veterinarians, researchers, physicians and public health authorities in a ‘one-health’ approach is crucial. Recognition of outbreaks in animals may provide an early warning to enable implementation of preventive measures to reduce human exposure, and for early recognition and prompt treatment of outbreaks in humans. Conversely, humans may act as sentinels of tularemia in a given area.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268814002398.
ACKNOWLEDGEMENTS
This work should be attributed to: the Department of Pathology and Wildlife Diseases, National Veterinary Institute, Uppsala, Sweden; Department of Biomedical Sciences and Veterinary Public Health, Swedish University of Agricultural Sciences, Uppsala, Sweden.
The study was supported by the Wildtech project (EU 7th Framework Program for Research and Technological Development, grant agreement no. 222633).
DECLARATION OF INTEREST
None.











