INTRODUCTION
One third of the world's population is infected with tuberculosis (TB) and about nine million people progress to TB disease every year, of which 2 million die [1]. Sub-Saharan Africa has the highest incidence with 290/100 000 person-years [Reference Frieden2]. It has been shown that poor performance status (rated using a scoring system) at the start of anti-TB treatment is a strong predictor of early death in pulmonary TB patients [Reference De Vallière and Barker3], but the relevance of habitual physical activity and fitness measured objectively has not been reported.
In patients with TB, the level of physical activity and fitness may be markers of the severity of disease, as well as determinants of recovery. In financially disadvantaged settings, a swift recovery from disease is crucial to enable the patient to return to work and thereby support the family again.
As part of a large nutrition study in TB patients, we measured fitness using heart rate (HR) response to a self-paced walk test and habitual physical activity using combined accelerometry and HR monitoring during >2 days of free living. In this paper we present data on the role of TB disease and HIV infection and other predictors on the level of physical activity and indicators of physical fitness.
METHODS
This paper is based on baseline data from a physical activity study nested in two large nutrition studies in TB patients [Reference Praygod4, Reference Praygod5] conducted during 2006–2009 in Mwanza City, Tanzania. For a limited period all TB patients admitted to Buzuruga Health Centre or Sekou Toure Hospital for initiation of TB treatment were invited to participate in the present substudy on physical activity. In addition to TB patients, non-TB neighbourhood controls were invited to participate in the present study to enable further comparisons. Physical activity was measured for all 156 TB patients and 26 controls who were invited to participate.
The control group was selected from the neighbourhood of TB patients. The TB patients participating in the nutrition studies were asked to provide detailed contact information about their local chief, and in cooperation with the chief a complete list of individuals from the same cluster of houses (10–20 houses), with the same sex and similar age (±5 years) as the TB patient, was compiled. From the list one individual was randomly selected by lottery, and invited to participate as control. None of the controls were household contacts of a TB patient, and to be eligible as a control, only adult (>15 years) participants were invited. Furthermore, the controls were not eligible if they had any history of TB, evidence of active TB (cough, intermittent fevers, excessive night sweating in the past 2 weeks, unexplained weight loss in the past month), were pregnant or lactating, suffering from other severe diseases, or non-residents of Mwanza City. Their HIV status did not influence eligibility. If the invited control was not eligible another was randomly selected from the list. The recruitment was performed on weekdays during daytime between 08:00 and 17:00 hours.
Prior to enrolment, the TB diagnosis in TB patients was based on microscopy using Ziehl–Nielsen stained specimen (for further details see [6]). Three additional sputum samples were collected in sterile universal bottles, and culture of Mycobacterium tuberculosis was performed on Lowenstein–Jensen solid media at the Zonal TB Reference Laboratory, Bugando Medical Centre. TB diagnosis was based on the culture result unless the sample was missing or contaminated in which case the microscopy result was used. TB patients with verified bacteria were diagnosed as having sputum smear-positive TB (PTB+). For TB subjects with a negative microscopy and culture (PTB–), the TB diagnosis was based on clinical suspicion, participant history, lack of clinical improvement after treatment with a broad spectrum of antibiotics, and X-ray results as suggested by the WHO [7]. TB patients aged <15 years, pregnant or lactating women, terminally ill patients (judged unlikely to survive >48 h), suffering from other severe diseases, as well as non-residents of Mwanza City, were excluded. Participants not capable of unassisted walking were excluded from the present study. Except for the diagnosis of TB, all participants (TB and non-TB) underwent the same measurements.
Height was measured to the nearest 0·1 cm and weight to the nearest 0·1 kg with the patient barefoot and wearing light clothing. Body mass index (BMI) was defined as weight/height2 (kg/m2). Venous blood was collected and analysed at the National Institute of Medical Research laboratory in Mwanza. All participants were tested for HIV using two rapid tests, Determine HIV 1/2 (Inverness Medical Innovations Inc., USA) and Capillus HIV-1/HIV-2 (Trinity Biotech plc., Ireland); if the results were discordant a confirmatory ELISA was performed. Cluster of differentiation 4 (CD4) counts were determined by flow cytometry after CD4 immunoflourochrome staining of the leucocytes (Partec FACS, Partec GmbH, Germany). Haemoglobin was determined and anaemia defined as haemoglobin <12·0 g/dl in women and <13·0 g/dl in men. Serum concentrations of the acute phase reactant alpha-1-acid glycoprotein (AGP) were determined at the Department of Clinical Biochemistry, Aalborg University Hospital, Denmark.
Upon arrival at the TB clinic during the morning, TB patients as well as controls were set up with a combined HR and movement (acceleration) sensor (Actiheart, CamNtech Ltd, UK), as described elsewhere [Reference Brage8]. In the present study, the monitor was attached to two ECG electrodes (Unomedical, Australia) on the chest with the right patch placed beneath the sternum, and the left patch at the medioclavicular line just beneath the left areola. The participants then completed a 300-m self-paced walk on a flat, firm floor, following which they relaxed for 2 min. Heart rate above sleep (HRaS) was calculated as the difference between observed HR and sleeping HR. Walk speed was calculated as distance (300 m) divided by time taken, from which HR economy (beats/metre) was derived as the ratio of walk HRaS over walk speed; this parameter was used as the main indicator of physical fitness and as a means of individually calibrating free-living HR data (see below). After the walk test, the monitor was set-up for long-term recording (collecting aggregated HR and acceleration data every 30 s) and each participant was thoroughly instructed in the local language (Kiswahili) by a Tanzanian TB clinical officer; including information about duration of monitoring (>2 days), basic functions of the monitor (HR, level of movement), and reminding participants to wear the monitor day and night, during showering and all other activities.
Upon reappearance at the clinic, data were downloaded and reviewed for lost data and erroneous information during the monitoring period. If obvious recording errors had occurred, the participant was asked to repeat the free-living monitoring.
The free-living HR and accelerometer data were cleaned and pre-processed using Gaussian process regression for inferring the latent HR from the potentially noisy HR data [Reference Stegle9]. If no movement or non-physiological HR was recorded for a prolonged period, the period was marked as non-wear time. Sleeping HR was determined from the days with complete (24 h) measurements as reported previously [Reference Brage8]. For the estimation of free-living physical activity energy expenditure (PAEE, in kJ/day per kg), we modelled the activity and HR data using branched equation modelling [Reference Brage10]. The estimation of activity intensity from cleaned free-living data was determined using group calibration equations for both accelerometry [Reference Brage11] and HR, the latter based on 1089 step tests conducted in a population sample from Kenya [Reference Christensen12]. Intensity time-series were expressed in multiples of resting metabolic rate [Reference Henry13], following which sedentary time and moderate-to-vigorous activity were quantified as proportion of time spent at intensities <1·5 metabolic equivalents (METs) and >3 METs, respectively.
Statistical analysis
All analyses were performed using Stata v. 11.2 statistical software (StataCorp LP, USA). The t test and one-way analysis of variance were used to test for differences in means, and the χ 2 test was used to test for differences in proportions. The parameters from the activity monitoring (i.e. sleeping HR, PAEE, sedentary physical activity) were used as dependent variables in linear regression models and with age, sex, TB status, HIV status, the acute phase reactant AGP and haemoglobin levels as independent variables. A normal haemoglobin levels was defined as >13 g/dl for men and >12 g/dl for women, while deficits of 0–2, 2–4, and >4 g/dl were used for increasingly severe levels of anaemia (Table 2, model 3), whereas AGP was dichotomized at 1·2 g/l to define elevated response (Table 2, model 4). Since PAEE was not normally distributed, log-transformation (naturaal logarithm to the base e) was used to achieve a normal distribution, thus the coefficient (eB) should be interpreted as a ratio. PAEE parameters from free-living measurements were adjusted for HR economy (meters walked/heart beat) derived from the walk test to account for the between-individual variance in HR–PAEE relations. Interaction between HIV, TB, age and sex was tested for. P values <0·05 were considered significant.
Ethical approval
Ethical permission was obtained from the ethics committee of the National Institute for Medical Research (NIMR) in Tanzania, and consultative approval was given by The Danish National Committee on Biomedical Research Ethics. Written and oral information was presented to all eligible participants by the health staff before written informed consent was obtained, and all participants were free to withdraw from the study at any time. Written consent was obtained from parents/legal guardians of any participants aged <18 years.
RESULTS
Of 156 TB patients and 26 non-TB controls recruited, 135 (86·5%) TB patients and 25 (96·2%) non-TB controls had complete data on physical activity and were included in the analyses. There were no differences between TB patients and controls with respect to age (mean difference of 1·4 years, 95% CI −4·1 to 7·0, P = 0·61) or sex (60·7% vs. 72·0% males, P = 0·29). Of TB patients, 94 (69·6%) had sputum-positive pulmonary TB (PTB+). More TB patients than controls had HIV (48·9% vs. 8·0%, P < 0·001), as previously reported [Reference Praygod4].
Background information, sleeping HR, walk test parameters as well as free-living physical activity data are shown in Table 1. With a walk speed of 1·3 m/s, the PTB+ patients had the fastest self-paced walk speed, compared to controls (1·0 m/s, 95% CI 0·9–1·1) and the PTB– patients (1·2 m/s, 95% CI 1·1–1·2), and, consequently, the PTB+ patients had the highest HRaS during the walk test with a 11·6 (95% CI 4·9–18·4) and 9·9 (95% CI 4·3–15·5) beats/min higher HR compared to the controls and the PTB– patients, respectively. The walking distance per heart beat, or the HR economy, based on this low-intensity exercise (walk test) was similar for controls and TB patients. During the non-clinical assessment of physical activity, average movement measured by the activity monitor's accelerometer was 0·11 m/s2 in controls, whereas the PTB+ and PTB– groups registered comparatively less movement, with measured levels of 0·06 (95% CI 0·05–0·07) and 0·04 (95% CI 0·03–0·06) m/s2, respectively. Similarly, median PAEE was lower in PTB– and PTB+ patients (29·5 and 37·6 kJ/day per kg, respectively), compared to controls (53·5 kJ/day per kg).
Table 1. Background information, walk-test data and habitual physical activity in 135 pulmonary tuberculosis patients and 25 controls
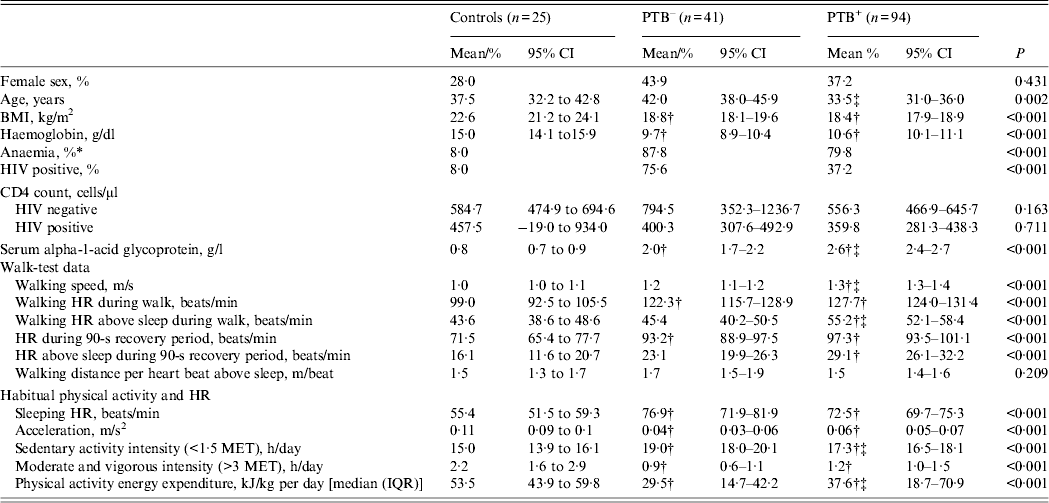
PTB–, Sputum smear-negative tuberculosis; PTB+, sputum smear-positive tuberculosis; CI, confidence interval; BMI, body mass index; HR, heart rate; MET, metabolic equivalent; IQR, interquartile range.
Data are mean (95% CI) or % unless otherwise stated.
* Anaemia defined as haemoglobin <12·0 g/dl in women and <13·0 g/dl in men.
† Denotes different from control.
‡ Denotes different from PTB–.
The associations between the TB disease and HIV infection and outcomes derived from the activity monitoring (i.e. PAEE, sedentary and moderate physical activity, sleeping HR) are shown in Table 2; with model 1 showing crude estimates, and models 2, 3 and 4 showing adjusted multivariable models. Patients with PTB– or PTB+ had a sleeping HR of 21·5 (95% CI 14·6–28·4) and 17·1 (95% CI 11·0–23·2) beats/min higher than controls, and these estimates were only marginally affected by age, sex and HIV infection. Moreover, HIV infection was associated with a higher sleeping HR (9·5, 95% CI 4·8–14·1 beats/min), but the difference decreased when adjusting for TB, age and sex (Table 2, model 2). While adjustment for haemoglobin halved the difference in sleeping HR for TB disease and HIV infection (Table 2, model 3), adjustment for AGP additionally attenuated the difference in PTB+ patients (Table 2, model 4). Although HIV infection was associated with reduced CD4 count (Table 1), further adjustment for CD4 count did not alter the results.
Table 2. The association between physical activity parameters and infection, i.e. tuberculosis and HIV, in 25 non-tuberculosis controls and 135 tuberculosis patients
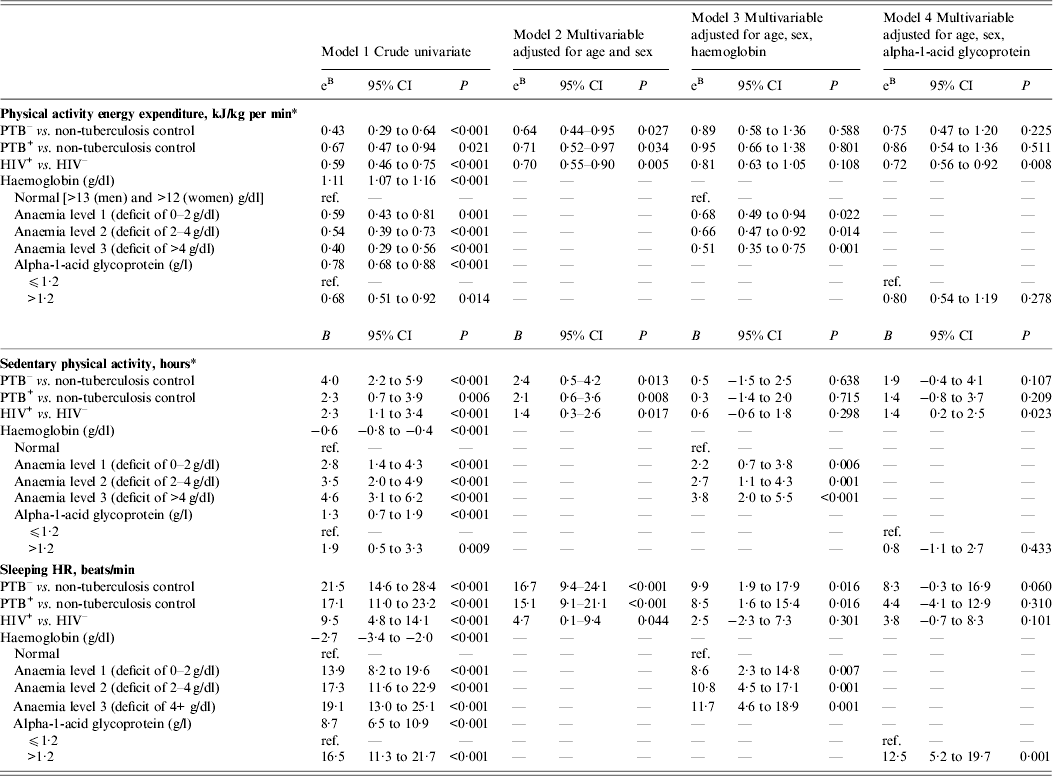
CI, Confidence interval; PTB–, sputum smear-negative tuberculosis; PTB+, sputum smear-positive tuberculosis; HIV+, HIV positive; HIV–, HIV negative Data are linear regression adjusted for age and sex (model 1), age, sex, HIV and tuberculosis (model 2), age, sex, haemoglobin, alpha-1-acid glycoprotein, HIV, and tuberculosis (model 3)
* Adjusted for HR economy (metres walked/heart beat).
Patients with TB disease spent 2–4 h (P < 0·01) more in sedentary activities compared to controls, and similarly, HIV infection was associated with more sedentary activity (2·3, 95% CI 1·1–3·4) compared to controls (Table 2). The increased time in sedentary activities was associated with anaemia, with those considered most anaemic (deficit >4 g/dl) spending almost 4 h more time on sedentary activities compared to those with normal haemoglobin levels (Table 2, model 3).
The average daily PAEE was less than half in the PTB– patients (eB 0·43, 95% CI 0·29–0·64) and less than two-thirds in PTB+ patients (eB 0·67, 95% CI 0·47–0·94) compared to controls (Table 2, model 1), and this association was only partly confounded by HIV infection (Table 2, model 2). Similarly, HIV infection was associated with 41% lower levels of activity compared to HIV uninfected participants (eB 0·59, 95% CI 0·46–0·75), which was only partly confounded by TB status. Anaemia was a strong mediator of the association between TB, HIV and physical activity (Table 2, model 3), while AGP primarily seemed to mediate the role of TB on physical activity (Table 2, model 4).
DISCUSSION
This is the first study using combined movement and HR monitoring in TB and HIV patients to assess the level of PAEE and other activity measures. We found that TB and HIV were associated with substantially higher sleeping HR, and a marked reduction in daily PAEE. Furthermore, anaemia was a strong mediator of the effects from TB and HIV, while the acute phase response primarily mediated the TB effect on the physical activity parameters.
The role of the acute phase response and the haemoglobin level indicates that the level of physical activity is inversely associated with severity of infection, while sleeping HR (the inverse of which is a marker of cardiorespiratory fitness) is positively associated with disease severity. Elevated serum AGP could account for the associations between TB, but not HIV, and performance. This is because TB is accompanied by a considerable acute phase response, whereas HIV per se is not. However, in this study HIV infection was associated with anaemia, which considerably reduces performance. Anaemia is known to be associated with infection, and lower levels in this study may therefore indicate either more severe stages of the infection or longer time living as treatment naive. Moreover, anaemia may decrease aerobic capacity, and therefore reduce capacity for performing daily activities as reflected in PAEE. The association between HIV and physical activity has previously been reported from a Kenyan retrospective cohort study assessing the impact of HIV/AIDS on individual labour productivity during disease progression [Reference Fox14]. The study reported a decline in work capacity by progressing HIV disease, and our observations on objectively measured activity levels corroborate these findings. In this study we consider any anaemia to be caused primarily by TB and/or HIV infection, although several other factors (e.g. nutritional characteristics, other infections) could similarly cause anaemia; however, such data were not available from the study.
Bed rest is associated with a decrease in muscle function [Reference Berg, Larsson and Tesch15], and since some of the TB subjects with more severe disease could have been immobilized for a prolonged period of time, this would most likely lead to decreased activity energy expenditure, simply due to inactivity and body tissue wasting. We have previously demonstrated that TB patients may have lost ∼10 kg of body weight due to the TB infection prior to enrolment of TB diagnosis, treatment and, hence, assessment of physical activity [Reference Praygod16]. Elevated sleeping HR is a common reaction to ongoing infections, both harmless and severe, but will reduce the span between sleeping and maximum HR, and hence reduce performance capacity.
The levels of physical activity and sleeping HR seem most severely affected in PTB– patients. Due to the lack of bacteria in the culture, it could be postulated that PTB– patients were less severely ill compared to PTB+. However, the group of PTB– patients consists of HIV uninfected individuals with early clinical manifestations of pulmonary TB as well as severely immunosuppressed HIV-positive infected patients, thus the PTB– group is very heterogeneous, and when adjusting the models for HIV infection, the difference between PTB– and PTB+ became smaller.
In our study, sleeping HR is associated with severe disease and could reflect increased metabolic demands caused by the infections, thus leading to higher resting energy expenditure. From a recent review it was reported that HIV and associated infections increase resting energy expenditure [Reference Kosmiski17], and it is plausible to assume that patients without access to sufficient nutrition intake will at some point reduce their activity level to sustain the total energy expenditure in an attempt to avoid negative energy balance. Furthermore, it has been reported from a HIV study from Kenya that disease progression leads to less physical activity and less labour productivity [Reference Fox14]. Although the lower activity level in HIV-infected individuals in our study was mediated by anaemia, the activity level remained reduced compared to HIV uninfected individuals.
This study has several limitations. The choice of control group was not optimal, since the control group was recruited in a different time period than the main study, but the controls were matched for age and sex which should limit confounding. In addition, average daily activity level observed in the control group was similar to levels reported from recent studies from Kenya [Reference Christensen12] and Cameroon [Reference Assah18]. Another limitation is the estimation of PAEE by combined sensing; although this is based on objective measurements, several stages of inference is necessary to estimate PAEE. The doubly labelled water technique is the gold standard for measuring the total energy expenditure in humans under free-living conditions [Reference Westerterp19, Reference Cole and Coward20], which, if combined with an assessment of resting energy expenditure, would provide a more accurate estimate of PAEE; however, this method is very expensive, and is difficult to carry out in large-scale studies. Furthermore, doubly labelled water cannot quantify subcomponents of physical activity like intensity, frequency and duration [Reference Johansson21], which may be deduced from combined movement and HR data. The impact on behaviour from awareness of wearing the device is potentially low, since it is worn below the clothes and not clearly visible. Increased awareness of being monitored could otherwise alter behaviour, i.e. the Hawthorne effect [Reference Wickström and Bendix22]. Last, the method of individual calibration could also be improved; a standardized and more intense test, e.g. a treadmill test, may be a better test than a walk test for calibrating the relationship between HR and physical activity intensity at the individual level [Reference Brage11], but this is less feasible in fragile populations, e.g. the elderly or the sick. We therefore opted for a self-paced walking test which may capture around 50% of the between-individual variance in the HR–PAEE relationship. All statistical models were adjusted with an individual calibration factor from the walk test but residual variance may still persist which will have the effect of attenuating associations towards the null; hence our results are likely to be conservative estimates of the differences in activity levels between these groups.
CONCLUSIONS
The level of PAEE and sleeping HR is highly influenced by severe infections, such as TB and HIV. The lower physical activity related to TB infection is explained by increased acute phase response and anaemia, whereas the association between HIV infection and physical activity was primarily mediated by anaemia. A better understanding of the physical activity level of individuals before and after TB treatment is necessary, since this could help enable faster recovery.
ACKNOWLEDGEMENTS
The authors thank all the health service providers at the TB clinics and the study participants involved in the study.
DECLARATION OF INTEREST
None.




