1 Introduction
According to the neurodevelopmental model of schizophrenia, psychotic symptoms emerge as a result of interactions between brain abnormalities established in early development and brain maturational events that occur much later [Reference Weinberger1]. Metals have well-known effects on neurodevelopment in children, some acting as essential nutritive elements and others as neurotoxicants [2–Reference Rodriguez-Barranco, Lacasana, Aguilar-Garduno, Alguacil, Gil and Gonzalez-Alzaga8]. For example, zinc (Zn2+) is essential in development of the nervous system, and is involved in neuronal proliferation and migration as well as modulation of synaptic activities and intracellular signaling pathways [Reference Adamo and Oteiza9]. Lead (Pb2+) interferes with intraneuronal gene transcription, affects hippocampal neurogenesis, and causes glial dysfunction in developing brain [Reference Bellinger10]. Furthermore, many essential elements, such as copper (Cu2+), manganese (Mn2+), and Zn2+, might exert toxic effects on brain at higher doses [11–Reference Guilarte14]. The link between exposure to different metals and adverse early neurodevelopmental outcomes is well known [2,4,6–8,15–Reference Brubaker, Schmithorst, Haynes, Dietrich, Egelhoff and Lindquist22]. However, the effect of metals on later developmental outcomes, such as schizophrenia, is still debated.
Beyond the general effect of metals on neurodevelopment, there are multiple intriguing links between several metals and schizophrenia (Table 1). Lead (Pb2+) and manganese (Mn2+) have been shown to cause alterations in neurotransmitters in the same manner that is often observed in patients with schizophrenia [23–Reference Guilarte, Opler and Pletnikov25]. For example, strong evidence links developmental exposure to Pb2+ with disrupted N-methyl-D-aspartate (NMDA) receptors function [Reference Guilarte, Opler and Pletnikov25], which can lead to NMDA receptor hypofunction with subsequent dysregulation of brain-derived neurotrophic factor (BDNF) signaling, synaptic function, and long-term potentiation [Reference Neal and Guilarte23, Reference Guilarte, Opler and Pletnikov25]. Furthermore, NMDA receptors, partly regulated by the tryptophan–kynurenine pathway, are essential during in utero brain development and influence synaptic formation and plasticity, cell proliferation, and cell migration during prenatal period [26–Reference Forrest, McNair, Pisar, Khalil, Darlington and Stone28].
Table 1 Clinical and neurobiological similarities of schizophrenia and altered balance of metals.

+ Evidence of association
* moderate association
** strong association.
Of note, individuals exposed to heavy metals or deficient in nutrient metals frequently experience neuropsychological deficits also observed in schizophrenia [5–Reference Salustri, Barbati, Ghidoni, Quintiliani, Ciappina and Binetti7,18,19,29]. Wilson’s disease, caused by a hereditary excess in Cu2+, frequently manifests with psychosis [Reference Akil and Brewer11]. Multiple reports have found different concentration of various metals, such as Cu2+, Mn2+, Zn2+, and cadmium (Cd2+) between patients with schizophrenia and healthy controls [30–Reference Pfeiffer and Iliev34]. Intriguingly, higher delta-aminolevulinic acid levels (marker of Pb2+ exposure) have been detected in mothers whose children later develop schizophrenia [Reference Opler, Brown, Graziano, Desai, Zheng and Schaefer35, Reference Opler, Buka, Groeger, McKeague, Wei and Factor-Litvak36].
The debate on the potential role of metals in schizophrenia is further complicated by methodological limitations of available studies. Importantly, metal exposure is mostly measured after disease development and is seldom determined during critical developmental periods. Studies that did measure metal exposure during critical developmental periods usually used indirect measures, such as maternal blood samples [Reference Opler, Brown, Graziano, Desai, Zheng and Schaefer35, Reference Opler, Buka, Groeger, McKeague, Wei and Factor-Litvak36]. Moreover, most studies relied on samples that do not reflect exact timing of exposure during the high-risk developmental period, and fall short of tracking change in exposure over time. To our knowledge, no prior studies have robustly studied the link between fetal or early childhood metal exposure and risk of psychotic illness in adulthood. Our primary objective was to determine whether the timing and dose of prenatal and early childhood metal exposure influences later development of schizophrenia and psychotic experiences.
2 Methods
In a proof-of-concept study, we analyzed shed deciduous teeth from nine individuals from the Genetic Risk and OUtcome of Psychosis (GROUP) [Reference Korver, Quee, Boos, Simons, de Haan and investigators G37] study with a DSM-IV diagnosis of schizophrenia and five healthy controls. GROUP is a prospective cohort of 1120 patients with psychotic disorders and 1648 controls and aims to investigate the genetic and environmental risk factors of psychosis in the Netherlands. A total sample size of 14 was estimated to be sufficient to detect a large effect size in the difference in mean log concentrations between cases and controls.
We investigated the association between exposure to metals, including manganese (Mn2+), lead (Pb2+), cadmium (Cd2+), copper (Cu2+), magnesium (Mg2+), and zinc (Zn2+), and intelligence quotient (IQ), syndromal schizophrenia as well psychotic experiences (as assessed by the Community Assessment of Psychic Experiences (CAPE) scale) [Reference Stefanis, Hanssen, Smirnis, Avramopoulos, Evdokimidis and Stefanis38]. Deciduous teeth from each subject were evaluated for pre- and postnatal metal exposure. We reconstructed the dose and timing of fetal and childhood metal exposures using a novel biomarker method named laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS), which has been described and validated in detail elsewhere [39–Reference Arora, Bradman, Austin, Vedar, Holland and Eskenazi45]. All teeth collected in the study were shed naturally and kept by the parents at home, as is often the case in some cultures. Teeth were stored dry in sealed containers of various types. The method for tooth analysis in this study excludes the external layers to avoid any contamination. Importantly, all analytical methods used here have been extensively validated and applied to samples stored over decades and archeological samples that are thousands of years old [Reference Arora, Austin, Sarrafpour, Hernandez-Avila, Hu and Wright44, Reference Austin, Smith, Bradman, Hinde, Joannes-Boyau and Bishop46]. Briefly, the method combines sophisticated histological and laser-based chemical analyses to precisely sample dentin layers corresponding to specific life stages, generating integrated, longitudinal, 1- to 2-week metal exposure estimates in pregnancy and during early childhood [Reference Hare, Austin, Doble and Arora39]. The time-varying difference between early-life (−4 to 6 months) metal concentrations, as measured in the tooth biomarker, and case/control designation was evaluated using a distributed lag model (DLM).
3 Results
Characteristics of the study participants and of the GROUP cohort are presented in Table 2. Mean [standard deviation, (SD)] age for the patients was 25.2 (1.9) years, and mean (SD) age for the control group was 28.0 ± 8.4 years. Mean (SD) duration of disorder was 3.8 (2.5) years.
Table 2 Demographic characteristics of all participants in GROUP study and those included in the pilot study.

IQ, intelligence quotient; PANSS, positive and negative syndrome scale; SD, standard deviation.
After stringent statistical correction, the longitudinal differences in log Pb2+ were generally estimated above zero, showing statistically significant higher early-life intake of Pb2+ among patients with schizophrenia compared with controls (Fig. 1A and B). The differences in log Mn2+ and log Cu2+ changed relatively linearly over time to postnatal negative values, indicating lower postnatal exposure to Cu2+ and Mn2+ in patients with schizophrenia than controls (Fig. 1A). The largest between group perinatal difference in Cu2+ and Mn2+ was observed six months postnatally (log difference case–control for Cu2+, −0.52, case/control ratio of 59% corresponding to 41% lower Cu2+ concentrations in cases compared to controls; log difference case–control for Mn2+, −0.42, case/control ratio of 66% corresponding to a 34% lower Mn2+ concentrations in cases compared to controls). There was a positive correlation between pre- and postnatal Pb2+ levels and CAPE score in adulthood (Fig. 1C). There was a negative correlation between Pb2+ levels and adult IQ (Fig. 1D), which was strongest during the second trimester of pregnancy (r = −0.39) and decreased gradually in the third trimester and postnatal period. There was a positive correlation between Mg2+ levels and IQ that peaked around birth (r = 0.28) and decreased postnatally (Fig. 1D).
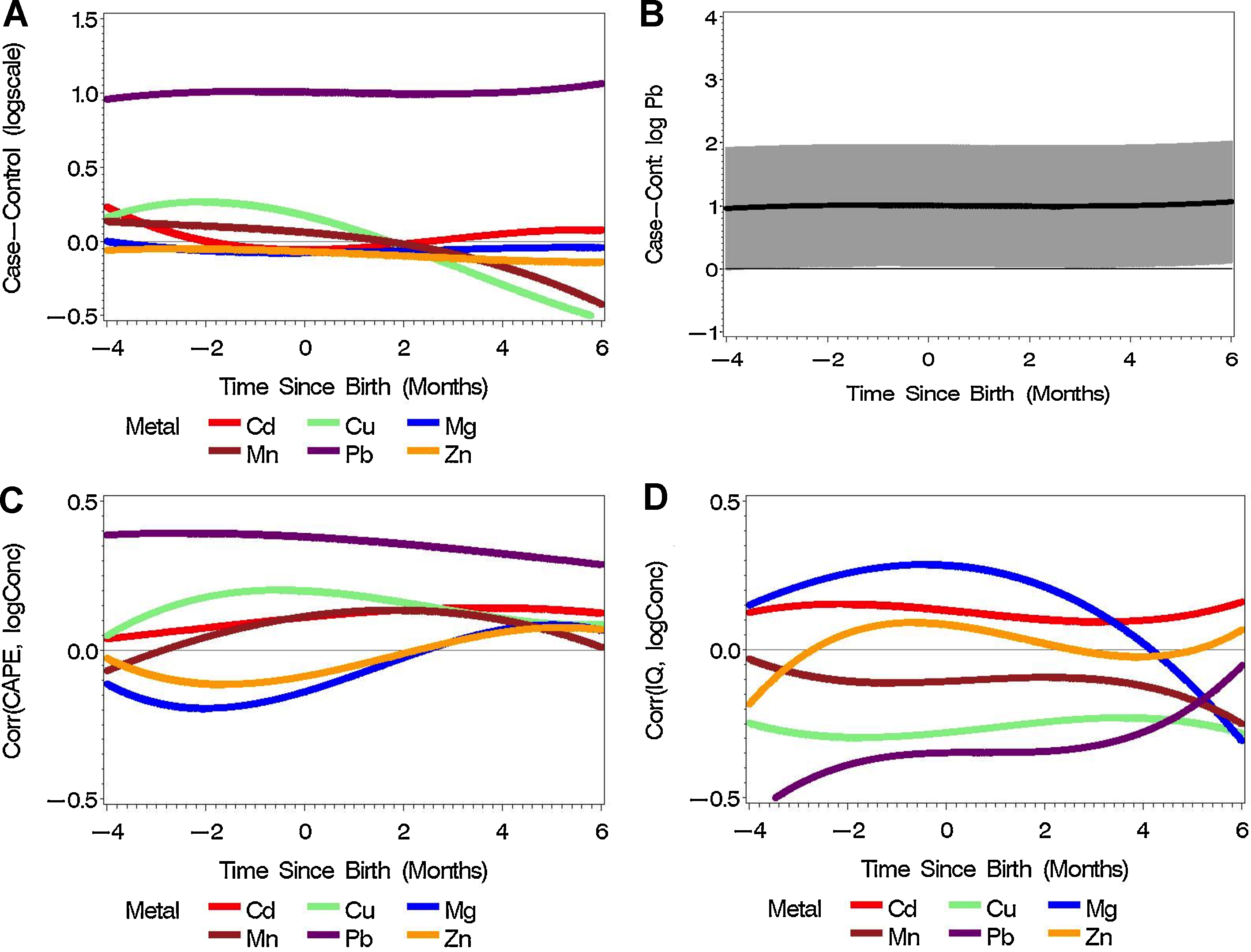
Fig. 1 Metal concentration in early life and adult psychosis and related phenotypes. (A) The difference of log concentrations between schizophrenia cases (N = 9) and controls (N = 5) for six metals during three developmental periods. (B) The differences of log concentrations between schizophrenia cases (N = 9) and controls (N = 5) for Pb2+ with Bonferroni-adjusted 95% confidence intervals. (C) The association between CAPE and perinatal log concentrations of six metals (N = 14). (D) The association between IQ and perinatal log concentrations of six metals (N = 14).
4 Discussion
Our proof-of-concept study is the first to use a tooth-matrix biomarker as a way to investigate the association between early-life metal exposure and long-term psychiatric outcomes. It provides initial support for the role of metal exposure during critical neurodevelopmental periods in early life and adult psychosis.
We believe that several characteristics make our study important. First, the study introduces a new method to reconstruct environmental exposures longitudinally from the second trimester and through the first year of life and it has the potential to transform research into environmental causes of mental disorders. The method is objective and therefore eliminates the possibility of bias in measuring environmental exposures. Second, correlations between metal exposures and severity of psychotic experiences ranged from 0.1 to 0.4, which is remarkable given the sample size, and that this is a unique longitudinal correlation across a 25-year period. Finally, unlike previous studies that used maternal samples as a way to measure fetal exposure, our method allows for direct measurement of exposure in utero, starting from second trimester of pregnancy. Larger sample sizes would definitely be beneficial as they would allow to increase power (which will lead to detection of more subtle statistical differences), and could help understand mechanisms better because they will allow for more sophisticated statistical analyses taking into account multiple metals.
We should also emphasize that the method is a highly sophisticated imaging technique that in contrast to all previous methods measures value of each metal for each individual hundreds of times across several months. Therefore, the tooth for each individual is represented by 120–180 distinct yet temporally connected values. Furthermore, although the sample size may seem small in clinical research, in molecular research, it is considered a moderate sample size. For example, PET imaging studies in human use comparable sample sizes [47–Reference Park, Hwang, Chu and Jung50]. Importantly, despite the small sample size, we were able to show a statistically significant effect for lead after a Bonferroni correction for multiple comparisons.
A clear pattern emerged from our results linking exposure to Pb2+ during early development with schizophrenia and psychotic experiences. Importantly, the association was both strong and statistically significant (i.e. the 95% CI for the ratios rarely included 1 and the correlation coefficient representing a ‘small’/‘medium’ effect size with 95% CI for the correlation coefficient rarely included zero). This confirms the findings of previous studies where more indirect measures of lead exposure were used to study the association between lead exposure during perinatal period and risk of schizophrenia. In two nested case–control studies, Opler et al. [Reference Opler, Brown, Graziano, Desai, Zheng and Schaefer35, Reference Opler, Buka, Groeger, McKeague, Wei and Factor-Litvak36] showed that higher delta-aminolevulinic acid (D-ALA) levels corresponding to ≥15 μg/dL blood lead level in the mother is associated with a twice increase in risk of schizophrenia in the offspring compared with a blood lead level of <15 μg/dL.
We also found an interesting association between higher lead levels and low IQ. While previous studies provided substantial evidence for the association between childhood lead exposure and low IQ [4,51–Reference Brown, Schaefer, Quesenberry, Liu, Babulas and Susser54], our study takes this evidence one step further. Some previous studies suggested that the effect of lead exposure on mental development was different depending on the trimester of pregnancy. This was based on maternal blood samples [Reference Hu, Tellez-Rojo, Bellinger, Smith, Ettinger and Lamadrid-Figueroa52, Reference Liu, Gao, Chen, Jing, Hu and Chen53]; but our study provides the first set of direct measurement of fetal in utero exposure. We found that lead levels during prenatal period had a significant negative correlation with IQ (r ∼ −0.5); but after reaching a plateau in the third trimester and first two months of life, the magnitude of correlation gradually decreased toward zero. This finding suggests that the prenatal period is the critical time where exposure to lead can have a substantial impact on intellectual development and that the lead impact tends to decrease as the brain gradually becomes more mature. It should be noted however that our sample was small, and therefore further replication of our finding is required.
We also found evidence of potential differential time-related relationship and critical windows of susceptibility for several other metals, such as Mg2+, Cu2+, and Mn2+, and adult outcomes. Although small sample size precludes any definitive interpretation of our findings, it is clear that determining the exact timing of exposure is critical, because exposure during different developmental periods confer different risks for adult outcomes.
Nevertheless, our findings should be interpreted in light of some limitations. First, our study was underpowered by design and therefore 95% CIs for estimates were very wide. Nevertheless and despite wide 95% CIs we could find a statistically significant effect of lead exposure on IQ and risk for psychosis. Furthermore, small sample size precluded statistical adjustment for important confounders, such as socioeconomic status and the interaction between metals. Third, although we tried to choose the most relevant metals for our study, there are several other metals that might be plausibly linked to abnormal neurodevelopment and consequently higher risk of psychosis. For example, iron regulates three key processes in brain development, namely energy metabolism, dopaminergic transmission, and myelination [Reference Georgieff55]. Importantly, studies suggest that iron deficiency is more prevalent in patients with schizophrenia than healthy controls [Reference Yanik, Kocyigit, Tutkun, Vural and Herken33]. Fourth, an inherent limitation of our method is that it cannot assess metal exposure during first trimester, which is considered another critical period in brain development. However, our novel biomarker provides the first set of direct in utero evidence and therefore is extremely valuable in assessing environmental exposures during pregnancy.
In summary, this study provides support for the role of metal exposure during critical early-life neurodevelopmental periods in psychosis. Our new biomarker represents a potential major advance in environmental science research in adult psychiatric disorders, since we can now objectively determine the temporal pattern of early-life environmental exposures. Further studies in a larger sample with a wider array of environmental exposures are required to fully elucidate the association between early-life environment and schizophrenia and related phenotypes in the population.
Financial disclosures
None declared.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
Dr. Velthorst was supported by (VENI) grant 916-15-005 from the Netherlands Organization for Scientific Research.








Comments
No Comments have been published for this article.