Methicillin-resistant Staphylococcus aureus (MRSA) is the leading cause of morbidity and mortality among antibiotic-resistant bacterial infections in the United States. Reference Klein, Mojica and Jiang1,2 Healthcare providers (HCP) are one of the known vectors that can transiently acquire MRSA from a patient who harbors the organism and subsequently transmit it to other patients in healthcare settings. Reference Blanco, O’Hara and Harris3–Reference Wilson, Hayman and Whitehouse8 HCP become transiently contaminated with MRSA following patient care 14%–20% of the time. Reference Morgan, Rogawski and Thom4,Reference Roghmann, Johnson and Sorkin5,Reference Snyder, Thom and Furuno9–Reference Pineles, Morgan and Lydecker11 The potential to transmit to other patients is increased if proper use of gloves and gowns does not occur or if the HCP is not wearing gloves and gowns when caring for a patient with MRSA. Reference Tomas, Kundrapu and Thota12,Reference Krein, Mayer and Harrod13
Acquisition rates of MRSA by HCP differ by the type of care provided. Reference Roghmann, Johnson and Sorkin5,Reference O’Hara, Calfee and Miller10,Reference Pineles, Morgan and Lydecker14 In previous studies, activities which require more direct patient contact, such as physical examination, bathing/hygiene, wound care, and maintenance of endotracheal tube, were more likely to be associated with HCP glove and gown contamination at the end of an HCP–patient interaction. Reference Roghmann, Johnson and Sorkin5,Reference O’Hara, Calfee and Miller10,Reference Pineles, Morgan and Lydecker11 However, in these studies, glove and gown culturing was conducted at the end of HCP–patient encounters and not after each individual patient care activity; thus, the true odds of HCP contamination by specific care activity were lacking. In addition, the frequency and factors related to transmission from contaminated HCP to subsequent patients are unknown.
In this study, we aimed (1) to determine the frequency of MRSA contamination of HCP gloves and gowns following each of 7 different care activities as well as the factors associated with transmission to the HCP by activity and (2) to determine the frequency of MRSA transmission from HCP to the subsequent patient (as represented by contamination of a proxy manikin) following direct care with an MRSA-positive patient if contact precautions are not used and hand hygiene is not performed.
Methods
Study design and participants
We conducted a prospective observational cohort study of MRSA-positive patients in the intensive care unit (ICU) and intermediate care units (IMCs) and their HCP to explore factors associated with patient-to-patient transmission of MRSA. The study had 2 objectives (Figs. 1 and 2), with the 2 associated substudies performed sequentially and thus 2 distinct cohorts. The first objective was to quantify the frequency of MRSA transmission from patients colonized and/or infected with MRSA to HCP gloves and gowns (surrogate outcome for transmission) immediately following a care activity and without performing hand hygiene. In this first objective, we also investigated factors associated with transmission by care activity. The second objective was to determine the frequency of MRSA transmission from HCP to the subsequent patient (as represented by contamination of a proxy manikin) immediately after a care activity with an MRSA-positive patient and without hand hygiene.

Figure 1. Study flow for objective 1.
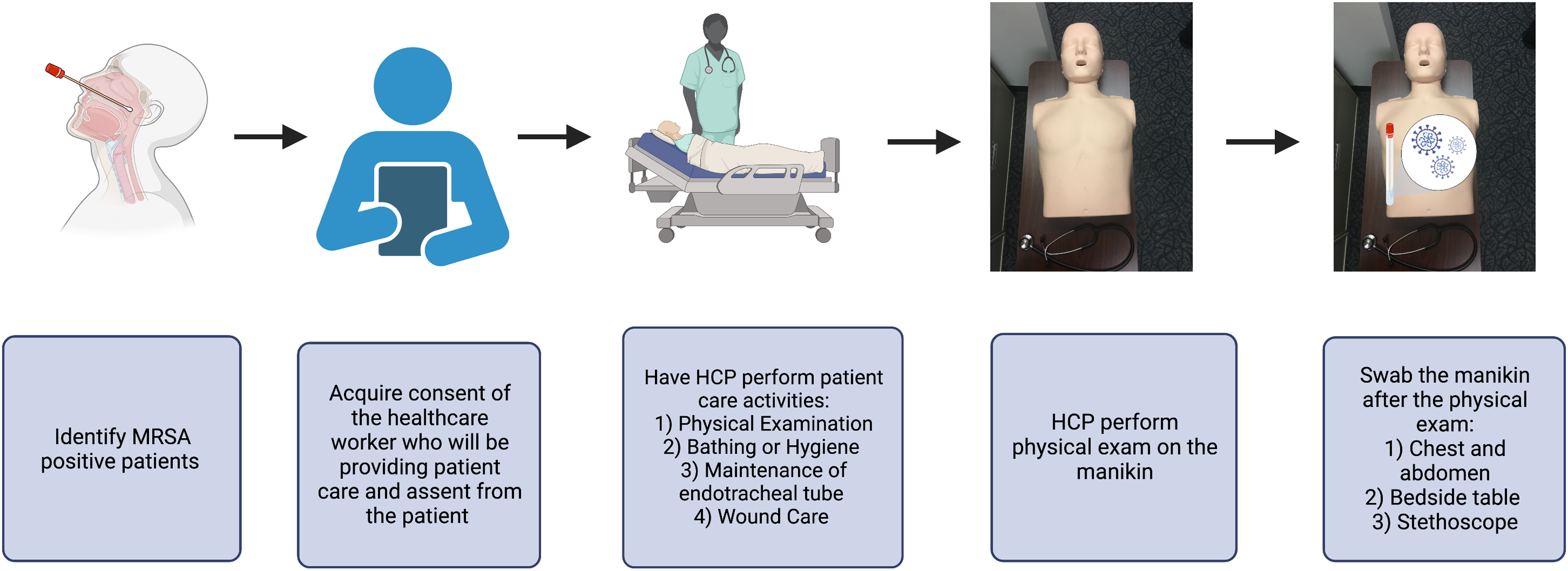
Figure 2. Study flow for objective 2.
Patients with a clinical or surveillance culture positive for MRSA in the previous 7 days and their HCP were recruited from the ICU and IMCs at the University of Maryland Medical Center between December 2018 and September 2021. Once enrolled, patients underwent surveillance cultures to confirm the current presence of MRSA, and random HCP who interacted with these patients were enrolled in the study before engaging in 1 of 7 specific care activities: physical examination, care of endotracheal tube, bathing/hygiene, wound care, glucose monitoring, administration of oral medications, and intravenous (IV) medication delivery or manipulation of IV tubing. These specific care activities were chosen for both objectives 1 and 2 based on literature review, which identified them as the activities most commonly associated with HCP contamination with MRSA. Reference Roghmann, Johnson and Sorkin5,Reference O’Hara, Calfee and Miller15 Research pertaining to objectives 1 and 2 was not conducted continuously because swabbing the gloves and gowns for objective 1 would affect the subsequent transmission to the manikin.
The University of Maryland Baltimore Institutional Review Board approved this study.
Data collection
Research staff were notified each day of patients on study units who had a positive clinical and/or surveillance culture for MRSA through an email alert linked to clinical microbiology results. Patients were enrolled in the study if they had had a culture positive for MRSA in the previous 7 days. Once enrolled and after obtaining patient assent, study cultures were obtained from the perirectal area, 2 skin sites (ie, one on the forearm and the other on the chest), and the nares to confirm MRSA status. The 2 skin sites, chest and forearm, were chosen to be consistent with previous studies. Reference O’Hara, Calfee and Miller10,Reference Jackson, Thom and Magder16,Reference O’Hara, Nguyen and Calfee17 Our sampling technique has been previously described. Reference O’Hara, Calfee and Miller10,Reference Jackson, Thom and Magder16,Reference O’Hara, Nguyen and Calfee17 Briefly, we swabbed the skin sites and the nares with a twirling motion using an Eswab (Copan Diagnostics, Murietta, CA), and the perirectal region was sampled by rubbing the skin around the rectum with an Eswab. Patient clinical characteristics, including the presence or absence of an artificial airway, indwelling urinary catheter, central line, chest tube, surgical drain, nasogastric (NG) tube, diarrhea, and wound were collected. International Classification of Disease, Tenth Revision (ICD-10) codes, age, sex, outcome after discharge, and race were abstracted from the medical records of each patient. The Elixhauser index, a validated comorbidity score, was calculated using the ICD-10 codes.
Objective 1: Patient-to-HCP transmission
We selected 7 common care activities to study based on literature review: physical examination, care of endotracheal tube, bathing or hygiene, wound care, glucose monitoring, administration of oral medications, and IV medication delivery or manipulation of IV tubing. Reference Roghmann, Johnson and Sorkin5,Reference O’Hara, Calfee and Miller15 HCP were approached prior to entering the room of a patient with the intent to perform routine patient care. HCP planning to perform one of the preidentified routine patient-care activities provided verbal consent for study enrollment. After consenting, and before performing one of these 7 activities, HCP were instructed to don new gloves and gown. Immediately following the planned activity with the patient, research staff swabbed HCP gloves and gown to determine the presence of MRSA. HCP role and duration of time spent on each activity were recorded.
Objective 2: HCP-to-subsequent patient transmission
For the second objective, we determined the frequency of MRSA transmission to a subsequent patient immediately following the planned activity with the patient and without performing hand hygiene. As in objective 1, different, random HCP were consented for participation prior to providing one of the preidentified care activities for a patient colonized or infected with MRSA. For this aim, we focused, a priori, on 4 care activities with the most direct HCP–patient contact and those that were more likely to be associated with HCP acquisition of MRSA in prior studies: physical examination, care of endotracheal tube, wound care, and bathing or hygiene. Again, HCP were instructed to don new gloves and gown before performing the specific care activity with the known MRSA-positive patient. Immediately after the care activity was completed and without removing their gloves and gown, the HCP was then instructed to follow a standard protocol and perform a mini physical assessment on a manikin simulating interaction with a subsequent patient. For this portion of the study, a manikin was placed on a bedside table at the edge of the room and set at the waist height of the participating HCP. Manikin, table, and a study-provided stethoscope were previously cleaned with Cavicide (Metrex, Romulus, MI) and allowed to dry. Just prior to the mini physical assessment, the table, the stethoscope, and the manikin chest and abdomen were sampled using environmental sampling swabs with neutralizing buffer (Puritan, Guilford, ME) as negative controls. After the HCP performed the mini physical assessment, sampling was performed again using the environmental sampling swabs, making sure to cover all surfaces, using a crisscross pattern on the manikin. Time spent performing the mini physical assessment was recorded.
Laboratory procedures
For patient samples and HCP glove and gown samples (first objective), swabs were cultured for the quantitation and presence of MRSA. E-Swab samples were spun in a vortexer, and 1:10 dilutions in Butterfield buffer were prepared. Aliquots of 100 µL dilutions were spread onto the MRSA CHROMagar (Becton Dickson, Sparks, MD) in triplicate and were incubated at 35°C ±2 for 24 hours. Rose- and mauve-colored colonies were counted from each plate, and the average count of colony-forming units per milliliter was recorded (CFU/mL). Swabs were also examined for the presence of MRSA by inoculating 50 µL tryptic soy broth (TSB) with 6.5% NaCl with the swab, which was then incubated at 35°C ±2 for 24 hours. Then, 100 µL broth was inoculated onto MRSA CHROMagar and incubated overnight. The presence of rose- and mauve-colored colonies was recorded. All MRSA isolates were confirmed as Staphylococcus aureus by latex agglutination and resistance to cefoxitin was confirmed by performing a Kirby Bauer disc-diffusion test.
For the second objective, manikin swabs were cultured for the presence of MRSA using TSB with 6.5% NaCl (Becton Dickson, Sparks, MD). TSB broth was incubated at 35°C ±2 for 24 hours, and 50 µL broth was then inoculated onto MRSA Chromagar (Becton Dickson, Sparks, MD). Plates were incubated at 35°C ±2 for 24 hours. Rose- and mauve-colored colonies were confirmed to be MRSA using susceptibility testing for MRSA using the Clinical Laboratory Standards Institute guidelines. 18
Statistical analyses
Demographic and clinical characteristics were calculated using frequencies with proportions and medians and interquartile ranges as appropriate. For objective 1, we estimated the following association with HCP glove or gown contamination: specific care interactions, HCP type, and patient clinical characteristics. Covariates that were significant at P < .05 in the analysis or demonstrated a 10% change when adjusted from the crude model were considered potential confounders in a multivariable model. We used a generalized linear mixed-effects model with a logit link function and a compound symmetry matrix to account for HCP correlation. For objective 2, we measured the association of manikin contamination with specific care interactions. Lastly, we estimated the overall risk of transfer of MRSA from a known MRSA-positive patient to a subsequent patient via a transiently contaminated HCP (in the absence of use of glove or gowns and no performance of hand hygiene) by identifying the frequency of HCP contamination from objective 1 and identifying the number of MRSA transfers onto the manikin in objective 2. All analyses were conducted using SAS version 9.4 software (SAS Institute, Cary, NC).
Sample size calculations
The sample size for each objective was calculated using proportions and confidence intervals based on prior literature of MRSA transfer frequency. Reference Ben-David, Mermel and Parenteau7,Reference O’Hara, Calfee and Miller15 For objective 1, we assumed a MRSA transfer rate of 14% from the patient to the gown and/or gloves of the HCP; for objective 2, we assumed a MRSA transfer rate of 5% from contaminated gown and/or gloves to the manikin and a sample sensitivity of 0.8. We estimated that the minimum number of HCP–patient interactions for objective 1 was 210, and for objective 2 the minimum number was 120 based on a 95% confidence interval with a width of 0.16. All sample size estimates were calculated using PASS version 13 software (NCSS, Kaysville, UT).
Results
Patient characteristics
In total, 144 unique patients with MRSA were enrolled in the study, including 98 patients for objective 1 and 85 patients for objective 2, with 39 patients contributing to both objectives. Moreover, 92 patients (63.9%) were male and 80 (55.6%) were white. Furthermore, 99 patients (68.6%) had an artificial airway. Also, 69 patients (47.9%) had a wound, 66 patients (45.8%) had a central line, 70 patients (48.6%) had an NG tube, and 60 patients (41.7%) had an indwelling urinary catheter (Table 1).
Table 1. Overall, Objective 1 and Objective 2 Patient Clinical and Demographic Characteristics

Note. IQR, interquartile range.
Objective 1: Patient-to-HCP transmission
Among the 98 MRSA-positive patients enrolled in this part of the study, we observed 333 individual–HCP patient interactions. Of the 333 HCP–patient interactions, physical examination (n = 57, 17.1%) was most frequent of the 7 care activities performed followed by medication delivery or manipulation of IV tubing (n = 55, 16.5%), bathing/hygiene (n = 52, 15.6%), glucose monitoring (n = 54, 16.2%), giving oral medications (n = 55, 16.5%), care of endotracheal tube (n = 47, 14.1%), and wound care (n = 13, 3.9%).
Among all 333 HCP–patient interactions, 54 HCP–patient interactions (16.2%) led to either HCP glove or gown contamination (Table 2); 49 HCP–patient interactions (14.7%) led to HCP glove contamination; and 16 HCP–patient interactions (4.8%) led to HCP gown contamination. Subsequent glove or gown contamination was associated with the specific care activities as follows: 23 (49%) of 47 endotracheal tube care interactions, 3 (23%) of 13 wound care interactions, 8 (14%) of 57 physical exam activities, 7 (13%) of 52 bathing or hygiene interactions, 7 (13%) of 55 oral medication activities, 3 (6%) of 54 glucose monitoring activities, 3 (5%) of 55 medication delivery interactions, and 3 (5%) of 55 IV medication delivery activities.
Table 2. Patient to HCP Transmission: Association Between MRSA Contamination on Gloves or Gowns and Clinical Characteristics and Type of HCP (N=333)

Note. HCP, healthcare personnel; IQR, interquartile range; NG, nasogastral; MD, medical doctor.
a P value calculated using χ2 test, Fisher exact test, Wilcoxon test, or Student t test.
b Column percentages were calculated for all variables.
Table 3 describes the multivariable model. Endotracheal tube care was significantly associated with an increased odds of glove or gown contamination compared to physical examination (unadjusted OR, 5.9; 95% CI, 2.3–15.1; adjusted OR, 4.06; 95% CI, 1.3–12.6).
Table 3. Patient to HCP Transmission: Adjusted Association Between Care Activities Performed and MRSA Contamination on Gloves or Gowns (N=333)

Note. HCP, healthcare personnel; CI, confidence interval; IV, intravenous.
a Other indicates allied health students.
Objective 2: HCP-to-subsequent patient transmission
In total, 85 MRSA-positive patients were enrolled in this part of the study. We observed 147 HCP–patient interactions: physical examination, care of endotracheal tube, bathing or hygiene, and wound care. Of 147 HCP–patient interactions, 15 (10.2%) led to subsequent manikin contamination. Of the 147 interactions and care activities performed in this part of the study, 55 (37.4%) were physical examinations, 55 (37.4%) were maintenance of endotracheal tube activities, 25 (17.0%) were bathing or hygiene activities, and 12 (8.2%) were wound care simulations. Subsequent manikin contamination was associated with the following specific care activities: 9 (16%) of 55 endotracheal tube care activities, 3 of 55 (5%) physical examinations, 1 of 25 (4%) bathing or hygiene simulation, and 2 of 12 (16%) wound care simulations. Contamination of the manikin occurred 7 (7.1%) of 99 times from nurses, 7 of 37 times from a respiratory technician (18.9%), 1 (14.3%) of 7 times from an MD/NP and 0 (0%) of 4 times from miscellaneous HCP.
Using findings from both objectives 1 and 2, we found that transmission from transiently contaminated HCP to a subsequent patient after performing a patient care activity may occur >60% of the time (10.2/16.2). Relative to a random activity, acquisition by the HCP and transfer to a subsequent patient may occur 1.6% of the time (0.102 from objective 2 × 0.162 from objective 1).
Discussion
MRSA contamination of HCP gloves or gown occurred 16.2% of the time after caring for a patient with MRSA. Among the patient care activities observed, performing endotracheal care was associated with the highest odds of contamination (49%, 23 of 47), whereas intravenous tubing and medication delivery had the lowest odds (5%, 3 of 55). Duration of activities was associated with activities performed (P = .03); however, it was not associated with MRSA contamination of the gown or gloves of HCP. In objective 2, subsequent MRSA contamination of a manikin after HCP–patient interactions (endotracheal care, physical examination, wound care, bathing/hygiene) with a MRSA-positive patient occurred 10.2% of the time. Together, these findings suggest that transmission from transiently contaminated HCP to a subsequent patient may occur greater than 60% of the time when contact precautions are not used after performing a high-risk activity and when subsequent hand hygiene does not occur. These data imply that foregoing contact precautions for patients with MRSA, particularly when hand hygiene rates are low, can result in patient-to-patient transmission, particularly during certain higher-contact clinical care activities with prolonged patient–healthcare personnel interactions.
In the first objective, we found that MRSA contamination of the HCP gloves or gown occurred 16.2% after HCP–patient interactions, consistent with other, similar studies. Reference Roghmann, Johnson and Sorkin5,Reference Snyder, Thom and Furuno9,Reference Tomas, Kundrapu and Thota12 Although other studies identified several care activities associated with HCP acquisition of MRSA from a MRSA-positive patient, the relative differences in transmission between various care activities has not been studied using a study design of sampling gloves and gowns after each individual care activity. Among 7 commonly performed care activities previously associated with transmission of MRSA to HCP, the frequency of HCP contamination was highest after care of endotracheal tube activity. In multivariable analysis, endotracheal tube care had the highest odds of MRSA contamination of HCP gloves or gowns (relative to performing physical exam) after adjusting for type of HCP and the number of medical devices. In prior studies, the type of HCP was an independent risk factor for transmission to gloves and gowns. O’Hara et al Reference O’Hara, Calfee and Miller10,Reference O’Hara, Nguyen and Calfee17 showed that respiratory technicians had the highest odds of MRSA glove and gown contamination. These data suggest that infection prevention activities aimed specifically at endotracheal care may have the highest impact on transmission reduction.
To our knowledge, our study is the first investigation of how often HCP gloves and gowns contaminated during care of an MRSA-positive patient may then lead to contamination of a subsequent patient. We used a simulated manikin exam to estimate subsequent transmission because using patients would be unethical. Contamination of the manikin occurred 10.2% of the time in our study. Together with findings from objective 1, this finding suggests that transfer of MRSA to the subsequent patient via transiently contaminated HCP can occur frequently from contaminated gloves and gowns that are not changed or from HCP hands or clothing if gloves and gowns are not worn for care of MRSA patients. HCP not wearing gloves could decrease this subsequent transmission rate by performing hand hygiene. Transmission rates from HCP clothing contamination alone independent of hand contamination (eg, scrubs) was not assessed in this study and needs to be assessed in future studies.
Our study had several limitations. We did not culture the entire glove or gown; we swabbed the midline of the gown and the fingers and the palm of the gloves. This may have led to an underestimate of glove and gown contamination rate. However, this swabbing technique is consistent with previous studies and helps to compare our findings with those studies. Reference O’Hara, Calfee and Miller10,Reference Jackson, Thom and Magder16,Reference O’Hara, Nguyen and Calfee17 Our study only involved certain healthcare provider patient activities. Additionally, the study was designed to maximize risk and thus likely represents the upper limit of plausible estimates of risks and potential benefits of contact precautions as an intervention. Finally, our findings may not be generalizable to settings other than large, urban teaching hospitals.
As healthcare institutions debate the advantages and disadvantages of contact precautions, our study provides additional data points that we hope will help guide evidence-based policies on contact precautions. We have identified certain high-risk activities that lead to higher potential contamination of hands and clothing if contact precautions are not used. We have also demonstrated the potential for subsequent transmission if contact precautions are not used.
Acknowledgements
We acknowledge Sujan Reddy and John Jernigan for their thoughtful comments and discussion on the development of this protocol.
Financial support
This project was funded by a CDC federal contract entitled Transmission of Antibiotic Resistant Bacteria from Patient-to-Patient in the Healthcare Setting and the Impact of Contact Precautions (BAA FY2018-OADS-01).
Competing interests
All authors report no conflicts of interest relevant to this article.








