Introduction
The ban in the use of antibiotic growth promoters (AGPs) in feed in many countries has encouraged animal scientists to search for alternative strategies to maintain gut health and, increase digestive efficiency and performance of broiler chickens. The use of probiotics, also termed direct-fed microbials (DFMs), in broiler diets is one such strategy that can reduce the incidence of enteric infectious diseases and increase broiler performance (Lee et al., Reference Lee, Lillehoj and Siragusa2010). Proposed modes of action whereby probiotics exert these effects have been reviewed, and these include improving the diversity and stability of intestinal microbiota, immunomodulation, competition for adhesion sites and production of antimicrobial agents (Patterson and Burkholder, Reference Patterson and Burkholder2003; Kabir, Reference Kabir2009; Lee et al., Reference Lee, Lillehoj and Siragusa2010). This is in contrast to AGPs that improve broiler performance by reducing overall microbial load in the digestive tract, decreasing competition for nutrients, and reducing microbial metabolites that depress growth (Dibner and Richards, Reference Dibner and Richards2005). However, concerns over the development of antimicrobial resistance have led European Union to ban in-feed AGPs and prompted research to find viable, sustainable alternatives to antibiotic use in animal feed.
Previous research has demonstrated that some Bacillus spp. are able to directly inhibit avian pathogenic Escherichia coli (APEC) as well as other microbes (Lee et al., Reference Lee, Lillehoj and Siragusa2010). These non-antibiotic strategies to reduce APEC are important to the poultry industry as APEC is responsible for diseases of high concern in the food chain, and which are also linked to reduced performance and profitability on farm (Lee et al., Reference Lee, Lillehoj and Siragusa2010).
In addition to influencing broiler performance, microbial activity in the caeca could further degrade any undigested phytate concentration in the digesta (Kerr et al., Reference Kerr, Classen and Newkirk1999), resulting in higher non-phytate P levels in the manure. The form of P in the manure has important environmental consequences, as a reduction in the proportion of manure P as phytate has been shown to be strongly correlated with increased manure water soluble P (Leytem et al., Reference Leytem, Plumstead, Maguire, Kwanyuen, Burton and Brake2008) and potentially result in greater P losses from surface applied manures that have not had sufficient time to react with soil and be converted into more stable forms (Penn et al., Reference Penn, Mullins, Zelazny, Warren and McGrath2004; Sturgul and Bundy, Reference Sturgul and Bundy2004). The aim of the present experiment was to compare the effects of supplementing broiler diets with a probiotic containing three Bacillus subtilis strains, or an antibiotic (zinc bacitracin; BMD), on the performance, intestinal mucosa-associated APEC levels, intestinal morphology and litter water-soluble phosphorus (WSP).
Materials and Methods
Birds and Housing
Experimental procedures were conducted in accordance with the Pretoria University Animal Ethics Committee guidelines and code of practice. Male broilers (Ross 308) were obtained as day-old chicks from a commercial hatchery, weighed and randomly assigned to 21 floor pens (50 chicks/pen). The floor pens were located in an environmentally controlled room. The chicks were subjected to 23 hours light: 1 hour dark for the first week and daylight hours were reduced thereafter to 16 hours per day. A commercial vaccination programme was used during the trial. Each pen of 3.6 m2 contained one bell drinker and one tube feeder. The concrete floor was covered in a 5 cm deep layer of fresh wood shavings. Room temperature was maintained at 32 ± 1 °C during the first week of the study and gradually decreased to 22 °C by the end of the fourth week. Mechanical fans in the walls of the shed controlled ventilation.
Diets and treatments
Three treatments were employed: an unsupplemented control diet; the same diet supplemented with the probiotic (Enviva ProTM 202 GT, Danisco Animal Nutrition, Marlborough, UK) at a level of 7.5 × 104 colony forming units (cfu) /g, or the basal diet supplemented with BMD (50g/tonne feed; Virbac Animal Health, South Africa).
Basal diets were formulated with corn and soybean meal (Table 1) and a two-phase feeding program was used, with the starter fed from 1–21 days and finisher from 22–42 days of age. Diets were formulated to meet or exceed the Ross 308 strain recommendations for major nutrients for broilers except available P (AvP) and Ca. The AvP and Ca concentrations were maintained at 1.5 and 1.1 g/kg below current Ross 308 recommendations and all diets were supplemented with phytase (500 FTU/kg diet, Phyzyme XP, Danisco Animal Nutrition, Marlborough, UK). Treatment diets were then created by supplementing basal starter and grower diets with BMD or the probiotic. Each of the three dietary treatments was randomly assigned to seven replicate pens.
Table 1. Composition and calculated analysis (g/100 g as fed) of the basal diet 1
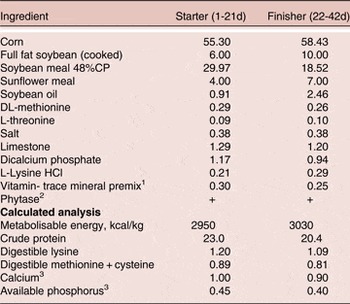
1 Supplied per kilogram of diet: antioxidant, 100 mg; biotin, 0.2 mg; calcium pantothenate, 12.8 mg; cholecalciferol, 60 µg; cyanocobalamin, 0.017 mg; folic acid, 5.2 mg; menadione, 4 mg; niacin, 35 mg; pyridoxine, 10 mg; trans-retinol, 3.33 mg; riboflavin, 12 mg; thiamine, 3.0 mg; dl-α-tocopheryl acetate, 60 mg; choline chloride, 638 mg; Co, 0.3 mg; Cu, 3 mg; Fe, 25 mg; I, 1 mg; Mn, 125 mg; Mo, 0.5 mg; Se, 200 µg; Zn, 60 mg.
2 Phyzyme XP 10000 TPT, Danisco Animal Nutrition, Marlborough, UK. The enzyme was included at a rate of 50g/tonne to supply a guaranteed minimum of 500 FTU/kg feed.
3 Includes the contribution from phytase of 0.11% Ca and 0.12% available P.
Avian Pathogenic E. coli Isolation and Enumeration Method
On d 42, one random bird from each replicate pen was sacrificed by cervical dislocation. Whole intestinal tracts were tied off at the oesophageal and caecal ends, placed in a sterile Whirl-PaK® bag with 5 ml of sterile physiological saline. Samples were immediately chilled by placing bags on ice and shipped overnight to the laboratory. Each intestinal sample was dissected to permit collection of approximately 15 cm of ascending duodenum, ~25 cm of the central small intestine (using Meckel's diverticulum as a reference point), collecting ~12.5 cm on either side, and ~15 cm of the ileum, collected in a proximal direction starting from the ileal-caecal junction. Sections were rinsed with sterile peptone water and cut lengthwise to expose the epithelial lining, combined in a filtered sterile Whirl-PaK® bag. A specified volume of peptone water was added to each bag and the contents masticated for 60 seconds. Processed samples were plated on CHROMagar™ agar (CHROMagar™, Paris, France) for the enumeration of E. coli. Plates were incubated at 37°C for 24 hours before counting. Typical E. coli colonies were picked and held in cryogenic storage at −80°C for further characterisation.
DNA Isolation and Multiplex PCR
DNA was isolated using a template preparation kit (Roche Diagnostics GmbH, Mannheim, Germany). Virulence gene profiles were determined by a multiplex PCR procedure (Skyberg et al., Reference Skyberg, Horne, Giddings, Wooley, Gibbs and Nolan2003). PCR products were identified by electrophoresis on a 3% Nu-Sieve agarose gel (BioWhittaker, Rockland, ME) and visualized by UV transillumination after staining in ethidium bromide solution. Gel images were captured using a Syngene BioImaging System (Cambridge, UK). A 100 bp DNA ladder (Bio-Rad, Hercules, CA) was incorporated on both sides of the gel as a standard. Specific gene content of E. coli isolates was determined by comparing the bands present for each sample to a positive control for each gene in question (Skyberg et al., Reference Skyberg, Horne, Giddings, Wooley, Gibbs and Nolan2003). The APEC genes screened included iss, iucC, tsh, cvaC and irp2. Isolates with two or more of the virulence genes were classified as APEC.
Intestinal Morphology Measurements
On d 42, two random birds from each replicate pen were sacrificed by cervical dislocation and sections from the middle of the duodenum and jejunum (about 5 cm in length) were excised and flushed with ice-cold saline, and immediately placed in Bouin's fluid. Samples were transferred into 70% ethanol after 24 h. Each fixed sample was then embedded into wax, and sectioned at a thickness of 7 µm, stained with alcian blue/haematoxylin-eosin and examined by light microscopy. Four intestinal segments were fixed in each slide and the slides were viewed on a Zeiss Axiophot microscope. Visual measurements of villus height and crypt depth were made on 10 villi at 100x and 200x magnifications using imaging software (Image Pro Plus, Version 4.1.0.9, Media Cybernetics, Silver Spring, MD). The variables measured were: villus height (the distance from the apex of the villus to the junction of the villus and crypt) and crypt depth.
Litter Mineral Contents
Litter was collected on day 41 from five locations within each pen. Samples within a pen were pooled and a representative sub-sample analyzed for litter total phosphorus (TP), water soluble phosphorus (WSP), nitrogen (N) and potassium (K). Analysis for TP and WSP were determined by standard AOAC (Reference Horwaitz2005) procedures 958.01 and 977.01, respectively. Total N was determined by standard AOAC (Reference Horwaitz2000) procedure 968.06. Potassium was determined using inductively coupled plasma according to Giron (Reference Giron1973).
Data Analysis
Pen means were used to derive performance data and manure P. For microscopic and intestinal APEC measurements, individual birds sampled from each pen were considered as the experimental unit. Bacterial numbers were logarithmically transformed to secure a normal distribution of the data (Yang et al., Reference Yang, Iji, Kocher, Mikkelsen and Choct2008). Data were subjected to one way ANOVA using the JMP 8.0 software and means were separated by Student's t-test. Differences were considered to be significant at P < 0.05, although P-values between 0.06 and 0.10 are shown in the text if the data approached significance.
Results and Discussion
Mortalities for control, BMD or probiotic treatments during the study were 14, 13 and 13%, respectively. The deaths were not associated with any specific treatment (P > 0.05), and were considered to be due to Sudden Death Syndrome.
The influence of treatments on the performance of broilers is summarised in Table 2. In general, body weight gain and feed conversion ratio (FCR) in the present study exceeded the Ross 308 strain standards. Over the 42-d trial period, BMD addition to the basal diets tended to improve the FCR by ~4 points with a numerical improvement in body weight gain of 60g. The improved broiler performance on BMD supplemented diets observed in the present study were comparable to a report by Engberg et al. (Reference Engberg, Hedemann, Leser and Jensen2000) in which BMD supplementation significantly improved body weight gain (~40g) with numerical improvements in FCR of five points. Other trials also showed slight improvement in FCR in diets supplemented with BMD (Stutz et al., Reference Stutz, Johnson and Judith1983; Waldroup et al., Reference Waldroup, Helling, Johnson, Tell, Primo, Cheng, Sims and Gerber1986). In contrast, a recent study by Teirlynck et al. (Reference Teirlynck, Bjerrum, Eeckhaut, Huygebaert, Pasmans, Haesebrouck, Dewulf, Ducatelle and Van Immerseel2009) reported no effect of BMD supplementation on 42 d broiler performance both in wheat/rye based diets and corn based diets. One of the proposed modes of action whereby AGPs improve performance is due to positive effects on the microflora in the proximal end of the small intestine, decreasing competition for nutrients and reducing microbial metabolites that can depress growth (Engberg et al., Reference Engberg, Hedemann, Leser and Jensen2000; Dibner and Richards, Reference Dibner and Richards2005). Therefore, it is plausible to suggest the performance effects of antibiotics were dependent on the microbial profile and stress status of the flock (Patterson and Burkholder, Reference Patterson and Burkholder2003), which may explain the variable performance responses to BMD observed in the literature.
Table 2. Influence of probiotic or zinc bacitracin (BMD) on the weight gain, feed intake and FCR of broilers fed corn/soy-based diets 1

NS, not significant; *P < 0.05; **P < 0.01.
a,b Means in a column not sharing a common superscript are significantly different (P < 0.05).
1 Each value represents the mean of seven replicates (50 birds per replicate).
2 Pooled standard error of mean.
The potential of using probiotics as an alternative to AGPs has been discussed extensively in the literature (Patterson and Burkholder, Reference Patterson and Burkholder2003; Ferket, Reference Ferket, Lyons and Jacques2004). Patterson and Burkholder (Reference Patterson and Burkholder2003) suggested that studies examining the effect of a probiotic should also include growth promotant antibiotics as a control treatment because stress status is important in detecting growth performance responses. In the current study, birds fed probiotic performed better on d 35 compared to both control and antibiotic supplemented diet. On d 42 no differences in performance of birds was observed between the antibiotic or probiotics treatments, but both BMD and probiotic treatments performed better than the unsupplemented control. This would suggest that probiotics can be used as an alternative to AGPs with better or similar broiler performance. These results are in agreement with the conclusion of Patterson and Burkholder (Reference Patterson and Burkholder2003) who stated that probiotics show promise as alternatives for antibiotics.
In the current study there was no treatment effect (P > 0.05) on villus height, crypt depth or height to depth ratio (Table 3). Miles et al. (Reference Miles, Butcher, Henry and Littell2006) reported no effect of BMD on villus height or crypt depth on intestinal morphology compared to control broilers fed a corn based diet. However, in the same study, birds given virginiamycin had shorter villi than birds given either unsupplemented diets or BMD, which suggests that the effect of antibiotic on intestinal histology may vary according to the antibiotic source.
Table 3. Influence of probiotic or zinc bacitracin (BMD) on villous height, crypt depth and height to depth ratio of the duodenum and jejunum of 42 day old broilers 1

NS, not significant.
1 Each value represents the mean of fourteen birds.
2 Pooled standard error of mean.
The effect of the dietary treatment on litter TP, WSP, N and K are shown in Table 4. An interesting finding of this study was that the WSP in manure was reduced (P < 0.05) by the inclusion of BMD in the diet versus the control diet or the diet supplemented with the probiotic. A previous study by Angel et al. (Reference Angel, Powers, Applegate, Tamim and Christman2005) investigated the effects of adding antibiotics to manure on the soluble P fraction. Results showed that freezing manure, or when an antibiotic was added, reduced WSP. In contrast, a rapid increase in WSP was observed when manure was incubated following excretion. This finding suggested that solubility of P in manures could be altered by microbial degradation of organic P fractions. The WSP content and the ratio of WSP:total P was also shown to be affected by the P composition of broiler litter (Leytem and Maguire, Reference Leytem, Maguire, Turner, Richardson and Mullaney2006). Leytem et al., (Reference Leytem, Plumstead, Maguire, Kwanyuen, Burton and Brake2008) subsequently showed that WSP in litter was strongly influenced by the phytate P content in litter, with steep linear reductions in WSP (as phytate P content) relative to total P in the litter increased. Therefore, dietary and other factors that alter the amount of phytate P excreted in the manure alter the solubility of the P therein. Reduced WSP in the present study following BMD supplementation suggest that the inclusion of antibiotics may reduce microbial degradation of phytate P in the digesta or litter, thereby reducing the soluble P fraction. The ban of AGPs from poultry diets may therefore have environmental implications, as a higher WSP content of litter has also been shown to increase P runoff after surface application of poultry manure (Kleinman et al., Reference Kleinman, Sharpley, Moyer and Elwinger2002).
Table 4. Effect of probiotic or zinc bacitracin (BMD) on litter total phosphorus (TP), water soluble phosphorus (WSP), water soluble phosphorus to total phosphorus ratio (WSP/TP), nitrogen (N) and potassium (K) at 41 d of age 1

1 % values on dry matter basis
2 Pooled standard error of mean
NS, not significant; *, P < 0.05.
a,b Means in a column not sharing a common superscript are significantly different (P < 0.05).
In the current study neither probiotic nor BMD had an effect (P > 0.5) on the number of intestinal mucosa-associated APEC (Figure 1). APEC is commonly present in the gut in low levels, but will negatively influence broiler performance when APEC levels are high (Yang et al., Reference Yang, Iji, Kocher, Mikkelsen and Choct2008). Previous research presented in literature has demonstrated that some Bacillus spp. are able to directly inhibit APEC as well as other microbes (Lee et al., Reference Lee, Lillehoj and Siragusa2010). The combination of the three strains of Bacillus subtilis used in the current study was selected based on their in vitro inhibitory effect on APEC or Clostridium perfringens. However, in the current study, the birds were perceived as healthy and the performance exceeded strain standards (Ross, 2007), which suggest that APEC levels in the gut can be considered low and may explain the lack of effect of probiotics on APEC. With regard to BMD, Yang et al. (Reference Yang, Iji, Kocher, Mikkelsen and Choct2008) observed an increase in the coliform population attached to the ileal mucosa in birds given BMD supplemented diet. They stated that BMD does not specifically target E. coli as this compound is active mainly against Gram-positive bacteria which may explain these findings.

Figure 1. Effect of probiotic or zinc bacitracin (BMD) supplementation on overall avian pathogenic E. coli count of intestinal mucosa of 42 day old broilers (n = 7)
Conclusions
Probiotic supplementation significantly improved broiler FCR compared to both the unsupplemented control group and birds receiving BMD as an AGP, and, as a result, can be considered as an alternative to AGPs. Under the conditions of the present study, probiotic supplementation had no effect on intestinal morphology, APEC or WSP.
Acknowledgments
The authors would like to thank Danisco Animal Nutrition (part of DuPont) for their financial support of this research.
Declaration of interest
The authors A. M. Amerah, P. W. Plumstead and C. Kromm are employees of Danisco Animal Nutrition. Danisco Animal Nutrition (part of DuPont) fully funded this research and own the rights to the probiotic blend Enviva ProTM 202 GT used in the experiment.







