Article contents
Design and mechanical properties simulation of fish scale-like intracranial thrombectomy stent
Published online by Cambridge University Press: 08 July 2020
Abstract
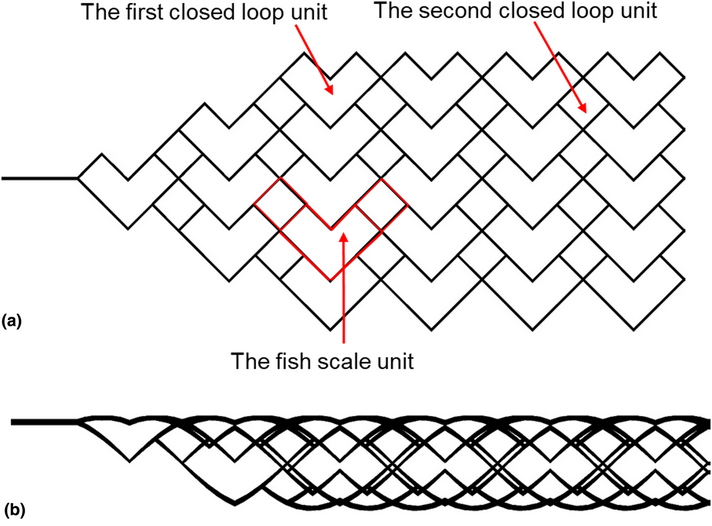
In recent years, intracranial thrombectomy stent has been an important method to treat ischemic stroke caused by acute thrombosis. In this paper, a new intracranial thrombectomy stent with a fish scale-like structure was designed and its mechanical properties were studied by a finite element method. The porosity of all stents was more than 80%. The space occupation ratio (SOR) of the stents increased linearly with the increase of strut thickness, while the strut width had little effect on SOR. The maximum equivalent stress and strain, the directional deformation and overall radial load of the stent decreased with the increase of strut thickness, however, the strut width has little impact on these parameters. The stents with 0.2 mm strut width and the thickness of 0.15 and 0.20 mm had better radial load performance, and the stent with 0.2 mm strut width and 0.15 mm strut thickness had better contact performance with the vessel wall and displayed better flexibility. Therefore, the present study provides a theoretical basis for the design of new intracranial thrombectomy stent.
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2020
Footnotes
These authors contributed equally to this work and should be considered co-first authors.
References
- 1
- Cited by





