Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Shibahara, H.
and
Taguch, H.
1991.
Hrem Study On The Interfacial Structure In Oxygen-Defective CaMnO3−x System.
MRS Proceedings,
Vol. 238,
Issue. ,
Parras, M.
Alonso, J.
González-Calbet, J.M.
and
Vallet-Regí, M.
1993.
Compositional variations and structural disorder in the BaMnO3− system.
Solid State Ionics,
Vol. 63-65,
Issue. ,
p.
614.
Burzo, E.
1996.
Perovskites I (Part a).
Vol. 27F1a,
Issue. ,
p.
135.
Burzo, E.
1996.
Perovskites I (Part a).
Vol. 27F1a,
Issue. ,
p.
147.
Burzo, E.
1996.
Perovskites I (Part a).
Vol. 27F1a,
Issue. ,
p.
194.
Nakano, Hiromi
Mori, Takanori
and
Kamegashira, Naoki
2002.
Structural analysis of La0.025Sr0.975MnO3 with a new layered structure.
Journal of Alloys and Compounds,
Vol. 343,
Issue. 1-2,
p.
179.
González-Jiménez, Irma N.
Torres-Pardo, Almudena
Sánchez-Peláez, Ana E.
Gutiérrez, Ángel
García-Hernández, Mar
González-Calbet, José M.
Parras, Marina
and
Varela, Áurea
2014.
Synthesis of 4H-SrMnO3.0 Nanoparticles from a Molecular Precursor and Their Topotactic Reduction Pathway Identified at Atomic Scale.
Chemistry of Materials,
Vol. 26,
Issue. 7,
p.
2256.
Hu, S
and
Seidel, J
2016.
Oxygen content modulation by nanoscale chemical and electrical patterning in epitaxial SrCoO3−δ(0 <δ≤ 0.5) thin films.
Nanotechnology,
Vol. 27,
Issue. 32,
p.
325301.
Heo, Yooun
Kan, Daisuke
and
Shimakawa, Yuichi
2018.
Nanoscale oxygen ion dynamics in SrFeO2.5+δ epitaxial thin films.
Applied Physics Letters,
Vol. 113,
Issue. 22,
Zhou, Catherine
De Graef, Marc
Dabrowski, Bogdan
Rohrer, Gregory S.
and
Salvador, Paul A.
2020.
Combinatorial substrate epitaxy investigation of polytypic growth of AEMnO3 (AE = Ca, Sr).
Journal of the American Ceramic Society,
Vol. 103,
Issue. 3,
p.
2225.
Sheeraz, Muhammad
Rashid, Mamoon Ur
Ali, Asad
Akram, Fazli
Lee, Ho Jeong
Choi, Jin San
Bae, Jong-Seong
Kim, Yong Soo
Shin, Young-Han
Ahn, Chang Won
and
Kim, Tae Heon
2021.
Stabilization of 6H-Hexagonal SrMnO3 Polymorph by Al2O3 insertion.
Journal of the European Ceramic Society,
Vol. 41,
Issue. 10,
p.
5155.
Li, Wei
Rothmann, Mathias Uller
Zhu, Ye
Chen, Weijian
Yang, Chenquan
Yuan, Yongbo
Choo, Yen Yee
Wen, Xiaoming
Cheng, Yi-Bing
Bach, Udo
and
Etheridge, Joanne
2021.
The critical role of composition-dependent intragrain planar defects in the performance of MA1–xFAxPbI3 perovskite solar cells.
Nature Energy,
Vol. 6,
Issue. 6,
p.
624.
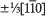 occurring between the face-sharing octahedra. Experiment by in situ observation during beam irradiation in an electron microscope indicates the process of the formation of the planar defect. A mechanism for the formation of the planar defect is proposed by which oxygen deficiency in face-sharing octahedra induces the displacement of SrO3 layers and oxygen octahedra which reduce the coordination number of Mn ions and the disruption of face-sharing octahedra.
occurring between the face-sharing octahedra. Experiment by in situ observation during beam irradiation in an electron microscope indicates the process of the formation of the planar defect. A mechanism for the formation of the planar defect is proposed by which oxygen deficiency in face-sharing octahedra induces the displacement of SrO3 layers and oxygen octahedra which reduce the coordination number of Mn ions and the disruption of face-sharing octahedra.