Article contents
Investigation of the persistent luminescence of LiBaPO4:Eu2+
Published online by Cambridge University Press: 04 February 2014
Abstract
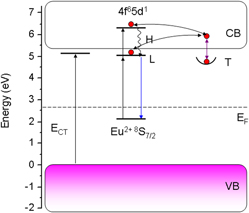
We investigated the persistent luminescence in europium-doped LiBaPO4. The persistent phosphors were synthesized via solid-state reaction method under mild reducing atmosphere. Its properties were investigated by x-ray diffraction, diffuse reflectance, photoluminescence, persistent luminescence, and thermoluminescence spectra. Under UV irradiation, broad-band persistent luminescence peaked at ∼470 nm was observed in the phosphors at room temperature. The effects of Eu2+ concentration on the persistent luminescence of LiBaPO4:Eu2+ were discussed. An energy level scheme was constructed to convey reasonable trapping and detrapping processes in the material.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 8
- Cited by




