Article contents
Paramagnetic defects in hydrothermally grown few-layered MoS2 nanocrystals
Published online by Cambridge University Press: 12 June 2018
Abstract
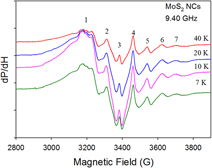
In the recent past, two-dimensional (2D) nanocrystalline (NC) transition metal dichalcogenides such as MoS2 received a great deal of attention due to their extraordinary physical properties. There has been a great interest to study the defects present in MoS2 NCs, which alter the material’s catalytic, electrical, and magnetic properties. This work reports paramagnetic point defects present in the hydrothermally grown 2H–MoS2 NCs. X-band electron spin resonance (ESR) spectroscopy has been used to identify the defects which contain unpaired electron spins in the as-prepared and Ar-annealed MoS2 NCs. At least seven ESR signals were detected originating from four inequivalent paramagnetic defect sites such as adsorbed oxygen species, sulfur vacancies, thio-, and oxo-Mo5+. Upon Ar-annealing, most of these defects did not survive, instead conduction ESR signal was observed. This work signifies the importance of employing ESR spectroscopy and broadens the knowledge in identifying the atomic defects in MoS2 NCs.
- Type
- Invited Paper
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 10
- Cited by




