Introduction
Amongst the organotin (OT) compounds, trisubstituted organotins (R3SnX), including tributyltin (TBT) and triphenyltin (TPT), have been extensively used as anti-fouling agents for the prevention of biofouling on ship hulls (Snoeij et al., Reference Snoeij, Penninks and Seinen1987; Blunden & Evans, Reference Blunden, Evans and Hutzinger1989; Bosselmann, Reference Bosselmann and de Mora1996). TBT and TPT pose deleterious hazards for non-target aquatic biota (Fent & Meier, Reference Fent and Meier1994; Ohji et al., Reference Ohji, Arai and Miyazaki2002, Reference Ohji, Arai and Miyazaki2003; Grzyb et al., Reference Grzyb, Rychlowski, Biegniewska and Skorkowski2003). Despite regulation of their use in antifouling paints, high concentrations of TBT and TPT persist in the aquatic ecosystem (Harino et al., Reference Harino, O'Hara, Burt, Pope, Chesman and Langston2002). In October 2001, the International Maritime Organization (IMO) adopted the International Convention on the Control of Harmful Antifouling Systems (AFS Convention), which prohibited the use of OTs as active ingredients in antifouling systems for ships. Following final ratification of the international restrictions on the use of OT-based antifouling compounds (in 2008 mostly), paint manufacturers have subsequently developed and deployed a variety of alternative products (Harino et al., Reference Harino, Mori, Yamaguchi, Shibata and Senda2005).
In Japan the new antifouling biocides substituted for OTs frequently include metal pyrithiones (PTs), such as copper pyrithione (CuPT2; 2-mercaptopyridine N-oxide copper salt) and zinc pyrithione (ZnPT2; 2-mercaptopyridine N-oxide zinc salt). Marinas and harbours represent high-risk areas for the accumulation of these PTs due to the high densities of boats on long-term moorings, as well as shore-based activities such as high-pressure hosing and washing of boats (Maraldo & Dahllöf, Reference Maraldo and Dahllöf2004). There are several studies which address the acute toxicity of CuPT2 and ZnPT2 to aquatic organisms, including algae, amphipods (Karlsson & Eklund, Reference Karlsson and Eklund2004; Eriksson Wiklund et al., Reference Eriksson Wiklund, Börjesson and Wiklund2006; Mochida et al., Reference Mochida, Ito, Harino, Kakuno and Fujii2006), sea urchins, mussels (Bellas et al., Reference Bellas, Granmo and Beiras2005) and marine fish (Mochida et al., Reference Mochida, Ito, Harino, Kakuno and Fujii2006, Reference Mochida, Ito, Harino, Onduka, Kakuno and Fujii2008), and these findings suggest that PTs can be toxic at environmentally relevant concentrations. Furthermore, six of the degradation products produced from CuPT2 and/or ZnPT2 by solar irradiation, namely 2,2′-dithio-bispyridine N-oxide (PT2), 2,2′-dipyridyl disulphide (PS2), 2-mercaptopyridine N-oxide (HPT), 2-mercaptopyridine (HPS), pyridine-2-sulphonic acid (PSA) and pyridine N-oxide (PO) (Sakkas et al., Reference Sakkas, Shibata, Yamaguchi, Sugaswa and Albanis2007), are as likely to be toxic to aquatic organisms as their parent compounds. However, there is little information regarding the toxicity of those compounds to aquatic organisms.
Marine bacteria are key elements of the biomass in coastal ecosystems and are vital for the recycling of nutrients. Indeed bacteria constitute the primary agents for the early transformation of organic matter and regeneration of nutrients and also serve as a food source for organisms at higher trophic levels. As such, they have an important ecological niche as decomposers and/or primary producers in coastal ecosystems worldwide. Consequently, monitoring microbial responses has been recommended as an early warning indicator of ecosystem stress, since microbes respond promptly to environmental perturbations (Griffiths, Reference Griffiths1983; Kim et al., Reference Kim, Koopman and Bitton1994). However, there have been few studies to date regarding the susceptibility of marine bacterial communities to pollutants (Konstantinou & Albanis, Reference Konstantinou and Albanis2003; Petersen et al., Reference Petersen, Pecharki and Scheie2004; Milenkovski et al., Reference Milenkovski, Bååth, Lindgren and Berglund2010), especially to antifouling agents (Blanck & Dahl, Reference Blanck and Dahl1996).
The pollution-induced community tolerance (PICT) approach has been used to assess minor effects of toxicants in biotic communities, and this method can establish causal linkages between contaminants and effects (Blanck, Reference Blanck2002). The PICT concept builds on the induced inter- and intra-specific selection of the most tolerant organisms to a toxicant, and on the establishment of mechanisms for detoxification (Corcoll et al., Reference Corcoll, Acuña, Barceló, Casellas, Guasch, Huerta, Petrovic, Ponsatí, Rodríguez-Mozaz and Sabater2014). The entire community may be restructured, present physiological alterations, and finally display an overall increase in tolerance to the toxicant, compared with a reference community (Tlili & Montuelle, Reference Tlili, Montuelle, Amiard-Triquet, Rainbow and Michele2011; Corcoll et al., Reference Corcoll, Acuña, Barceló, Casellas, Guasch, Huerta, Petrovic, Ponsatí, Rodríguez-Mozaz and Sabater2014). Defining PICT, as proposed for microbes here, therefore enables investigation of the cause-effect relationships between toxicant exposure and community response (Corcoll et al., Reference Corcoll, Acuña, Barceló, Casellas, Guasch, Huerta, Petrovic, Ponsatí, Rodríguez-Mozaz and Sabater2014) and is potentially a useful tool to assess the sensitivity of coastal microbial communities. R2A agar is generally used for enumerating heterotrophic organisms and was developed by Reasoner & Geldreich (Reference Reasoner and Geldreich1985) for bacteriological plate counts of treated potable water. A low nutrient medium, such as R2A agar, in combination with a lower incubation temperature and long incubation time stimulates the growth of stressed and chlorine-tolerant bacteria (Reasoner & Geldreich, Reference Reasoner and Geldreich1985). Nutritionally rich media support the growth of fast-growing bacteria but may suppress slow-growing or stressed bacteria found in treated water. In comparison, studies with nutritionally rich media (Standard Methods Agar) such as Tryptone Glucose Yeast Extract Agar or Plate Count Agar, R2A agar has been reported to improve the recovery of stressed and chlorine-tolerant bacteria from drinking water systems (Means et al., Reference Means, Hanami, Ridgway and Olson1981; Fiksdal et al., Reference Fiksdal, Vik, Mills and Staley1982; Kelly et al., Reference Kelly, Justice and Nagy1983). This agar is recommended in Standard Methods for the Examination of Water and Wastewater and can be used in pour, spread plate and membrane filter methods (Franson, Reference Franson1998). Like the bacteria in potable water, the bacteria inhabiting ambient seawater are also heterotrophic organisms. Therefore, in the present study, we considered this agar to be a suitable choice for elucidating the susceptibility of marine bacterial communities to antifouling biocides.
The objective was to compare the susceptibility of colonies of coastal microbes to CuPT2 and ZnPT2, over 7 days of culturing. This included comparison of toxicities between parental compounds and their degradation products, and also a comparison of toxicities between these new antifouling biocides with toxic organotin predecessors, TBT and TPT. The results form the basis of discussion on the fluctuation of abundance of native marine bacterial communities as well as on the biological impact of antifouling biocides.
Materials and methods
Site description
Seawater including marine bacteria was collected in sterilized bottles from the Imazu Coast, located in Hyogo Prefecture, central Japan. The seawater samples were immediately brought back to the laboratory and stored at 4°C until they were used in the experiment testing the inhibitory effects of bacterial growth by antifouling chemicals.
Experimental solution
CuPT2 and ZnPT2 were provided by Yoshitomi Fine Chemicals (Osaka, Japan). PT2 was purchased from Across Organics (Morris Plains, NJ, USA). PS2, HPT, HPS, PO, TBTCl (tributyltin chloride) and TPTCl (triphenyltin chloride) were purchased from Tokyo Kasei Kogyo Company (Tokyo, Japan). PSA was purchased from Wako Pure Chemicals Industry (Osaka, Japan). The chemical structures of the metal pyrithiones, their degradation products, and TBT and TPT are shown in Figure 1. The R2A agar, purchased from Nihon Pharmaceutical Co., Ltd (Tokyo, Japan), contained yeast extract, 0.5 g, peptone, 0.5 g, casamino acids, 0.5 g, glucose, 0.5 g, soluble starch, 0.5 g, K2HPO4, 0.3 g, MgSO4 7H2O, 0.05 g, sodium pyruvate, 0.3 g, and agar, 15 g, in one litre of distilled water. NaCl was added to the agar media to match the culture salinity conditions of the marine bacteria with that of the ambient water.

Fig. 1. Chemical structures of the metal pyrithiones, copper pyrithione (CuPT2) and zinc pyirithione (ZnPT2), and their degradation products, 2,2′-dithio-bispyridine N-oxide (PT2), 2,2′-dipyridyl disulphide (PS2), 2-mercaptopyridine N-oxide (HPT), 2-mercaptopyridine (HPS), pyridine-2-sulphonic acid (PSA), and pyridine N-oxide (PO), and organotins, tributyltin (TBT) and triphenyltin (TPT).
Stock solutions of 1000 mg l−1 of CuPT2, ZnPT2, the other six compounds, TBT and TPT were made by dilution with a non-toxic organic dissolvent, dimethyl sulphoxide (DMSO), which was autoclaved. The stock solutions were further diluted with DMSO to make working solutions of 1, 10 and 100 mg l−1, of which 1 ml of each was added to 100 ml of R2A agar media in a 200-ml Erlenmeyer flask and stirred to achieve dissolution before starting toxicity tests. Control solutions were also made using 1 ml DMSO in 100 ml of R2A agar media.
Each exposure experiment consisted of a control (0 mg l−1) and four test concentrations of each compound (0.01, 0.1, 1 and 10 mg l−1). Seawater including marine bacterial communities was mixed with autoclaved seawater to represent dilutions of 10−1, 10−2, 10−3 and 10−4.
Toxicity experiments
The comparative toxicity of antifouling compounds on bacteria was conducted using a modified form of the test used to assess the ecological effects in the risk assessment programme of the Organization for Economic Cooperation and Development (OECD, 1997).
One millilitre of seawater, or diluted seawater, containing marine bacterial communities was poured into a sterile Petri dish. R2A agar medium including each concentration of CuPT2, ZnPT2, PT2, PS2, HPT, HPS, PSA, PO, TBT and TPT was poured into the Petri dishes containing these seawater samples. These preparations were mixed thoroughly by rotating the plate several times. When the media had solidified, the plates were inverted and incubated at 25°C for 7 days in the dark in an incubator. The bacterial colonies were counted after 7 days of incubation. Each toxicity test was conducted in duplicate. The results were expressed as relative abundance (% of control). The CFU (colony forming unit) of each treatment was calculated relative to the control as follows:
 $${\rm Relative\ CFU} (\% \ {\rm of\ control}) = \displaystyle{{{\rm Number\ of\ CFU\ of\ each\ compound}} \over {{\rm Number\ of\ CFU\ of\ each\ control}}} \times {\rm 1}00$$
$${\rm Relative\ CFU} (\% \ {\rm of\ control}) = \displaystyle{{{\rm Number\ of\ CFU\ of\ each\ compound}} \over {{\rm Number\ of\ CFU\ of\ each\ control}}} \times {\rm 1}00$$The EC50 values were then determined from regressions of log transformation plots of the dose-response data (Nweke et al., Reference Nweke, Alisi, Okolo and Nwanyanwu2007).
Results
Toxicity of CuPT2 and ZnPT2 to marine bacteria
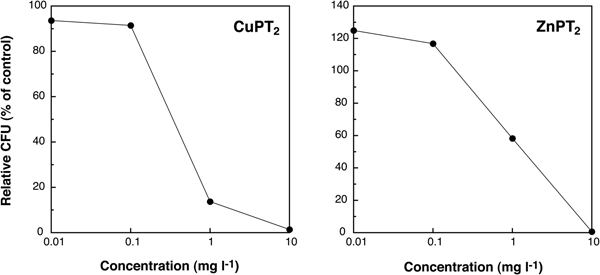
The relative CFU (% of control) of CuPT2 at a concentration of less than 0.1 mg l−1 was 91.5–93.6%, and the rate of colony formation decreased dramatically at concentrations greater than 0.1 mg l−1 (Figure 2). The colony forming rate of samples exposed to ZnPT2 was higher than controls (>100%) at 0.01 and 0.1 mg l−1 and decreased, progressively, at concentrations greater than 0.1 mg l−1 (1.0–10 mg l−1). The EC50 value of CuPT2 (0.3 mg l−1), was lower than that of ZnPT2 (1.4 mg l−1). These results indicate that CuPT2 was more toxic to marine bacterial communities than ZnPT2.

Fig. 2. Changes of relative colony forming units (CFU) (% of control) of individuals exposed to CuPT2 and ZnPT2 after 7 days of culturing.
Toxicity of the degradation products of PTs to marine bacteria
The relative CFU of four of the degradation products of CuPT2 and ZnPT2 decreased gradually with increasing concentration, except for PO and PSA when growth reductions were <20% across the exposure range (Figure 3). Hence the EC50 value of PO and PSA could not be calculated because the values of relative CFU of those chemicals were not below the required 50% reduction in growth needed for calculation. The EC50 value of HPS and PS2 was 8.1 and 3.7 mg l−1, respectively. The EC50 value of HPT and PT2 was 0.8 and 0.5 mg l−1, respectively, and these values were lower than those of their parent chemical, ZnPT2. These findings suggest that the toxicities of HPT and PT2 to marine bacteria were higher than that of ZnPT2.

Fig. 3. Changes of relative colony forming units (CFU) (% of control) of individuals exposed to PT2, PS2, HPT, HPS, PSA, PO after 7 days of culturing.
Toxicity of TBT and TPT to marine bacterial communities
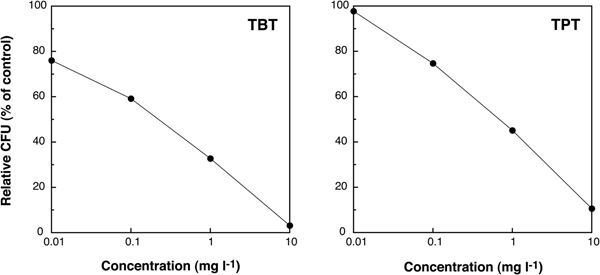
The values of relative CFU decreased in a dose-dependent manner with increasing concentration of these two organotin compounds (Figure 4). The EC50 value of TBT was 0.2 mg l−1, lower than that of TPT (0.7 mg l−1), suggesting that the toxicity of TBT to marine bacteria was higher than that of TPT.

Fig. 4. Changes of relative colony forming units (CFU) (% of control) of individuals exposed to TBT and TPT after 7 days of culturing.
Discussion
The results in the present study of microbial communities indicate that the toxicities of alternative antifouling biocides, such as CuPT2 and ZnPT2, that have been used as substitutes for TBT and TPT, are almost the same as those of the triorganotins. This suggests that marine bacterial communities are equally susceptible to metal PTs in the environment as they are to TBT and TPT exposure during their life history. It is also noteworthy that the toxicities of some of the degradation products of PTs were the same as or higher than those of their parental compound ZnPT2 and also TPT. Remarkably little is known about the toxicities of triorganotins to bacteria, other than the work of Avery et al. (Reference Avery, Miller, Gadd, Codd and Cooney1991) who showed that EC50 values (50% inhibition of growth after 7 days of incubation) ranged from 0.01 to 10 mg l−1. The EC50 of marine bacterial communities examined in the present study was thus comparable to the range shown by the bacteria in the study of Avery et al. (Reference Avery, Miller, Gadd, Codd and Cooney1991).
CuPT2 is readily converted from ZnPT2 in the presence of Cu ions and is the most stable PT (Nakajima & Yasuda, Reference Nakajima and Yasuda1999), and as such is recognized as a potentially hazardous compound to marine organisms. Currently, as most booster biocides are used as the combination of Cu-based antifoulants, the trans-chelation of the pyrithiones might be occuring in the ambient seawater. A few previous studies on toxicities of PTs to marine organisms such as bacteria have shown that the EC50 values (e.g. inhibition of bioluminescence) were of the order of 0.12 mg l−1 for CuPT2 and 0.08 mg l−1 for ZnPT2 (Zhou et al., Reference Zhou, Okamura and Nagata2006). CuPT2 was more toxic than ZnPT2 to the sea bream Pagrus major and the toy shrimp Heptacarpus futilirostris (96-h LC50 of CuPT2 and ZnPT2: 9.3 and 98.2 µg l−1 for P. major, and 2.5 and 120 µg l−1 for H. futilirostris respectively) (Mochida et al., Reference Mochida, Ito, Harino, Kakuno and Fujii2006). CuPT2 was also found to be more toxic to the copepod Tigriopus japonicus than ZnPT2 (24-h LC50: 41 µg l−1 and >500 µg l−1, respectively) (Yamada, Reference Yamada and Konstantinou2006). Furthermore, our previous study on Japanese killifish indicated that the toxicity levels of CuPT2 were higher than those of ZnPT2 (Ohji & Harino, Reference Ohji and Harino2017).
In the present study, the EC50 value of CuPT2 was 0.3 mg l−1, much lower than that of ZnPT2 (1.4 mg l−1), confirming that CuPT2 is more toxic than ZnPT2 to marine bacteria: The colony forming rate after exposure to ZnPT2 was more than 100% of that of controls at concentrations of 0.01 and 0.1 mg l−1 of ZnPT2. Only at a concentration of 10 mg l−1 ZnPT2 was a significant decrease in growth observed.
In contrast to these bacterial trends, CuPT2 has been reported to be less toxic to the marine diatom Skeletonema costatum (72-h EC50 was 28.4 µg l−1) than ZnPT2 (72-h EC50 was 2.1 µg l−1) (Yamada, Reference Yamada and Konstantinou2006). CuPT2 appears to be less toxic to the algae Chaetoceros gracilis and S. costatum, because Cu is an essential element for their growth (Hall & Anderson, Reference Hall and Anderson1999). The stimulatory effect observed with marine bacterial populations exposed to ZnPT2 at low concentrations may be attributed to the use of Zn as a trace element by marine bacterial communities. The same condition, perhaps a form of hormesis, was reported by Nweke et al. (Reference Nweke, Alisi, Okolo and Nwanyanwu2007), who a used dehydrogenase assay to assess the tolerance to Zn2+ by pure cultures of Salmonella species isolated from river sediment. The dehydrogenase activity was found to be slightly stimulated at 0.02 mM Zn2+ and progressively inhibited at concentrations greater than 0.2 mM (0.4–1.0 mM). Zinc is associated with a number of processes essential for growth and metabolism in bacteria (Choudhury & Srivastava, Reference Choudhury and Srivastava2001). Although Zn is an essential element, it is also an inhibitor of respiratory electron transport systems of bacteria and mitochondria, where high concentrations of Zn are inhibitory, affecting many crucial functions (Kasahara & Anraku, Reference Kasahara and Anraku1974; Rogers & Li, Reference Rogers and Li1985; Pérez-Garcia et al., Reference Pérez-Garcia, Codina, Cazorla and de Vicente1993; Beard et al., Reference Beard, Hughes and Poole1995; Choudhury & Srivastava, Reference Choudhury and Srivastava2001). Therefore, the inhibition of growth of bacterial populations observed in this study is considered to be consistent with the reported toxic effect of Zn at high concentrations (Ji & Silver, Reference Ji and Silver1995).
Comparing toxicities of CuPT2 and ZnPT2 between marine and freshwater organisms indicates that freshwater species are more sensitive to the toxicity of metal pyrithione than are marine species (Madsen et al., Reference Madsen, Samsøe-Petersen, Gustavson and Rasmussen2000; Yamada & Kakuno, Reference Yamada and Kakuno2002; Mochida et al., Reference Mochida, Ito, Harino, Kakuno and Fujii2006; Ohji & Harino, Reference Ohji and Harino2017). PTs are used as antifoulants not only in saline habitats but also in freshwater environments, such as lakes and rivers. Furthermore, ZnPT2 has been widely used as a biocide in anti-dandruff shampoos in many countries for years (Shuster, Reference Shuster1984) and may enter aquatic habitats from domestic waste in addition to leachate from antifouling paints used on boats and ships. Therefore, it is important to consider the risk of ZnPT2 to freshwater bacteria as well as that to marine bacteria.
The results of the present study confirm that PTs can affect marine bacterial communities. But to evaluate risk fully it is necessary to combine toxicity data with investigations into the ambient levels of metal pyrithione in marine ecosystems. However, there are few such studies on the occurrence of PTs in nature, and these have usually focused on CuPT2 in sediment (Harino et al., Reference Harino, Midorikawa, Arai, Ohji, Cu and Miyazaki2006). To understand the risk of these biocides to aquatic ecosystems more fully, further research on the use, occurrence and toxicity of these biocides in aquatic environments, both freshwater and marine, is needed. In the present study, the toxicity of a range of antifoulants to marine bacteria decreased in the sequence TBT>CuPT2>PT2>TPT>HPT>ZnPT2>PS2>HPS. In contrast, in our previous study comparing the toxicity of the same biocides to the freshwater fish Oryzias latipes, the metal pyrithiones CuPT2, followed by ZnPT2, were most deleterious whilst TBT, TPT and degradation compounds of PTs were less toxic (Ohji & Harino, Reference Ohji and Harino2017). The displayed differences in toxicity of these antifouling compounds may be habitat dependent and/or species specific, and this field clearly warrants further investigation.
Photolysis is a significant factor in the degradation of CuPT2 and ZnPT2 in natural environments (Armbrust, Reference Armbrust2000; Turley et al., Reference Turley, Fenn and Ritter2000; Maraldo & Dahllöf, Reference Maraldo and Dahllöf2004; Harino et al., Reference Harino, Mori, Yamaguchi, Shibata and Senda2005). It seems likely that degradation compounds will have a lesser impact on aquatic organisms than the parent compounds. However, Bellas et al. (Reference Bellas, Granmo and Beiras2005) demonstrated that the toxicity of ZnPT2 decreased but did not disappear entirely after exposure to direct sunlight. PTs may persist in the marine environment where the influence of the light is limited, such as shaded waters and sediments in marinas and harbours (Maraldo & Dahllöf, Reference Maraldo and Dahllöf2004). Here, for example, ZnPT2 may accumulate in the sediment as manganese pyrithione and CuPT2 (Galvin et al., Reference Galvin, Mellado and Neihof1998), and impact on the aquatic ecosystem. Although to date there are only a few reports regarding the occurrence of degradation products of PTs, i.e. PS2 in seawater (Mochida, personal communication), our previous study demonstrated that various sub-lethal biological effects on Japanese killifish (e.g. abnormalities in respiration and swimming behaviour and decreased hatchability) can be caused by exposure to degradation compounds of CuPT2 and ZnPT2 (Ohji & Harino, Reference Ohji and Harino2017). However, it is still not fully understood how these degradation products affect O. latipes, or their mode of action in marine bacteria.
In conclusion, our results indicate that in terms of the viability of marine bacterial communities, the toxicities of new antifouling biocides such as CuPT2 and ZnPT2 that are used as substitutes for TBT and TPT are almost the same as the banned chemicals they are replacing. These findings suggest that marine microbial communities may be at reasonably high ecological risk from the effects of CuPT2 and ZnPT2 exposure as well as those from TBT and TPT exposure. It was noteworthy that the toxicities of some of the degradation products of PTs are the same as, or higher than, those of their parental compound (ZnPT2) and TPT. Thus, new pyrithione-based antifouling biocides cannot be assumed to be totally safe alternative biocides to OTs, as both types of compound appear equally capable of inducing disturbances in marine microbial communities.
Acknowledgements
Thanks are due to Yoshitomi Fine Chemicals for provision of Cu and Zn pyrithione.
Financial support
The present study was partially supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Nos. 18780141, 19681005 and 20688008).