Introduction
With growing globalization, the occurrence of marine invasions by non-indigenous species is increasing, raising concerns about their potential consequences on invaded environments. Invasive predators can have particularly strong impacts on invaded ecosystems via competition with native predators and through predation on native prey species and communities (Bruno et al., Reference Bruno, Fridley, Bromberg, Bertness, Sax, Stachowicz and Gaines2005). Two recent predatory invaders along European shores, the Asian shore crab Hemigrapsus sanguineus (De Haan, 1835) and the Asian brush-clawed crab Hemigrapsus takanoi (Asakura & Watanabe, 2005) may have the potential for strong effects on native competitors and prey species. Native to the western Pacific, the newcomers were first sighted in European coastal waters in the 1990s. Since the first records in France and the southern Netherlands, both species have spread rapidly and their current distribution reaches along the continental European coast from France to the Baltic Sea. More recently, individuals of both species have also been reported from Great Britain (Epifanio, Reference Epifanio2013; Seeley et al., Reference Seeley, Sewell and Clark2015; Wood et al., Reference Wood, Bishop, Davies, Delduca, Hatton, Herbert and Clark2015; Karlsson et al., Reference Karlsson, Obst and Berggren2019).
In invaded ecosystems the two Hemigrapsus species can commonly be found in sympatry with the native European shore crab Carcinus maenas (Linnaeus 1758) (Breton et al., Reference Breton, Faasse, Noël and Vincent2002; Dauvin et al., Reference Dauvin, Rius and Ruellet2009; Karlsson et al., Reference Karlsson, Obst and Berggren2019). Where they occur together, the invasive Hemigrapsus spp. probably compete with native C. maenas for shelter and food and often locally replace C. maenas as the dominant species, especially on hard substrates (Dauvin et al., Reference Dauvin, Rius and Ruellet2009; Van den Brink et al., Reference van den Brink, Wijnhoven and McLay2012; Landschoff et al., Reference Landschoff, Lackschewitz, Kesy and Reise2013). Due to their high abundance and effectiveness as predators, the invasive crabs can have detrimental effects on local prey communities, as indicated by studies conducted in the USA where H. sanguineus has been introduced as well (Brousseau et al., Reference Brousseau, Filipowicz and Baglivo2001; DeGraaf & Tyrrell, Reference DeGraaf and Tyrrell2004; Brousseau & Baglivo, Reference Brousseau and Baglivo2005; Tyrrell et al., Reference Tyrrell, Guarino and Larry2006). Despite the potentially strong effects of the two invasive crabs on native biota in Europe, our current knowledge about the feeding behaviour and prey preferences of the invasive crabs is mainly limited to studies from North America where C. maenas and H. sanguineus are invasive. In contrast, studies on the feeding preferences of H. sanguineus and potential competition with native C. maenas in European coastal waters are scarce. Even less is known about the feeding ecology of H. takanoi which has hardly been studied outside of its native range. The existing studies from native and invaded regions on prey preferences of the three crab species suggest that all three species are generalist omnivores, feeding predominantly on small molluscs, crustaceans, polychaetes and algae (Ropes, Reference Ropes1968; Ledesma & O'Connor, Reference Ledesma and O'Connor2001; Baeta et al., Reference Baeta, Cabral, Marques and Pardal2006; Doi et al., Reference Doi, Iinuma, Yokota and Watanabe2009; Klassen, Reference Klassen2012; Blasi & O'Connor, Reference Blasi and O'Connor2016). Experiments from the USA and Japan suggest that H. sanguineus may have a more herbivorous diet than C. maenas (Lohrer et al., Reference Lohrer, Whitlatch, Wada and Fukui2000; Griffen, Reference Griffen, Galil, Clark and Carlton2011) but that they both prefer animal over plant tissue when given a choice and are therefore possible competitors (Brousseau & Baglivo, Reference Brousseau and Baglivo2005; Griffen, Reference Griffen, Galil, Clark and Carlton2011). Lipid analyses from crabs collected on Helgoland in the European North Sea suggest that a similar pattern may occur in invasive H. sanguineus in Europe and experimental studies have indicated that all three crab species overlap in their use of mussels as diet (Jungblut et al., Reference Jungblut, McCarthy, Boos, Saborowski and Hagen2018; Bouwmeester et al., Reference Bouwmeester, Waser, van der Meer and Thieltges2020). However, experimental studies on prey preferences of the two invasive crab species in comparison with native crabs, also including other prey species than mussels, are missing from European locations to date.
The present study experimentally investigated prey preferences of invasive (H. sanguineus and H. takanoi) and native (C. maenas) intertidal crabs in the European Wadden Sea. Using laboratory experiments offering four types of prey species (bivalves, gastropods, amphipods and algae) either separately (no-choice treatments) or at the same time (choice treatments), we assessed and compared the prey preference of each of the three species to estimate the potential for resource overlap and subsequent competition between the native and invasive crabs. Based on previous studies, mainly from the invasion in the USA, we hypothesized that the native C. maenas prefers animal food over plant material (Ropes, Reference Ropes1968; Elner, Reference Elner1981) and that H. sanguineus has a generally more herbivorous diet than C. maenas (Lohrer et al., Reference Lohrer, Whitlatch, Wada and Fukui2000; Griffen, Reference Griffen, Galil, Clark and Carlton2011; Jungblut et al., Reference Jungblut, McCarthy, Boos, Saborowski and Hagen2018). In addition, we expected the diets of the two Hemigrapsus species to be more similar to each other than to C. maenas, resulting from their close relation and origin.
Materials and methods
Crab collection
Crabs were collected by hand during low tide in spring 2019 in the south of the island of Texel in the Dutch Wadden Sea. Sampling took place along the dike in Mokbaai, a tidal flat close to the NIOZ Royal Netherland Institute for Sea Research (53°00′13.0″N 4°46′50.5″E), and along the dike between the ferry harbour at Mokbaai and the NIOZ (53°00′15.0″N 4°46′54.8″E). After sampling, all crabs were brought to a climate chamber at NIOZ, where their gender, potential claw damage and size (carapace width) were determined. In order to reduce variability associated with gender and size, only male crabs between 15–20 mm carapace width and with intact chelae were kept for further experiments as this was the dominant size range of both Hemigrapsus spp. during the time of sampling. Similar-sized Carcinus maenas were used because equally sized competitors generally have the greatest impact on foraging behaviour (Smallegange & Van der Meer, Reference Smallegange and van der Meer2006). This is also indicated by similar mussel size preferences of the three species at similar body sizes (Bouwmeester et al., Reference Bouwmeester, Waser, van der Meer and Thieltges2020). At a size of 15–20 mm, individuals of both Hemigrapsus spp. are adults, while in C. maenas they are still juveniles (Crothers, Reference Crothers1967; McDermott, Reference McDermott1998). Each crab was only used once in an experiment and recently moulted (soft) crabs were excluded due to their potentially different feeding behaviour (Baeta et al., Reference Baeta, Cabral, Marques and Pardal2006).
Crabs were kept at a constantly maintained temperature of 15–17 °C, corresponding to typical local summer water temperatures (van Aaken, Reference van Aken2008), the season where all three species show highest abundance. They were housed separately for each species in large flow-through plastic tanks of 36 × 26.5 × 25 cm (L × W × H) and provided with filtered seawater as well as aeration and small flowerpots and stones for shelter. For the duration of the experiments, crabs were kept at a day : night cycle of 12 h with dimmed light from 8 pm until 8 am and fed a diet consisting of crushed blue mussels (Mytilus edulis).
Prey collection
Prey species were chosen based on their high relative abundance at the study site, as well as on the basis of previous research, which suggested them to be important for the diet of all three crab species: blue mussels (M. edulis), sea lettuce (Ulva lactuca), periwinkle snails (Littorina littorea) and amphipods (Gammarus locusta). Other highly abundant potential prey such as brown algae (Fucus spp.) were not consumed in pilot experiments and therefore not investigated in this experiment. For reasons of standardization and because crabs were shown to be able to consume these size classes, only mussels and snails between 5 and 8 mm were used (Gerard et al., Reference Gerard, Cerrato and Larson1999; Bourdeau & O'Connor, Reference Bourdeau and O'Connor2003; Perez et al., Reference Perez, Carlson, Shulman and Ellis2009). For amphipods, no indication for size preference could be found in the literature and we used individuals between 5 and 15 mm which had been consumed by crabs in pilot experiments.
Mussels were collected at a nearby groyne on the south-west side of the island of Texel (53°01′21.9″N 4°42′26.2″E). They were manually removed from the substrate, stripped of sand and other attached material and their maximum shell length was measured to the nearest millimetre using callipers. Mussels of suitable size (5–8 mm) were kept in a flow-through aquarium of 25 × 15 × 10 cm (L × W × H) provided with filtered seawater, air and fed with algal feed (GroTech Plankto Marine P) every two days. Small periwinkles were sampled in Mokbaai and on the dike next to the ferry harbour by carefully removing them from stones. Their total shell length was measured to the nearest millimetre. Periwinkles of suitable size (5–8 mm) were kept in a plastic tank of 21 × 13.5 × 12 cm (L × W × H) filled with filtered seawater and fed a diet consisting of U. lactuca. Water was replaced every two days and any dead or damaged individuals were removed. Amphipods were collected in Mokbaai by turning stones lying in small puddles, removing the top layer of sand, and subsequently sieving the sand over 4 mm and 500 μm sieves using filtered seawater to separate the amphipods. In addition, amphipods attached to Fucus brown algae were obtained by rinsing algae collected from the dike with filtered seawater. Attached amphipods were washed into the water, which was then sieved through a 200 μm sieve from which they were collected. Amphipods (5–15 mm) were kept in plastic tanks of 21 × 13.5 × 12 cm (L × W × H) filled with filtered seawater and provided U. lactuca as food. To prevent mass dying due to possible infections, only 100–200 individuals were kept together in one tank. Water was replaced every day and any dead individuals or other pollutants were removed. Finally, sea lettuce was collected at the NIOZ Seaweed centre and kept in aerated filtered seawater. The sea lettuce in the centre originates from wild sources from the surroundings of NIOZ. All prey species were kept in a climate chamber at a constantly maintained temperature of 15–17 °C.
Experimental design
To investigate prey preferences of C. maenas, H. sanguineus and H. takanoi, single individuals of each species were either offered six mussels, six amphipods, six periwinkles or ~0.5 g of sea lettuce (‘no-choice treatments’) or they were offered all four prey species at the same time (‘choice treatments’). In addition, we ran controls without a crab for each prey treatment to identify potential algal growth/loss or mortality of other species not caused by crab predation during the experiment. The wet weight of sea lettuce leaves was measured to the nearest 0.1 mg after removing excess water using paper towels. Each prey species was held separately in small plastic containers containing aerated seawater. Due to the ability of periwinkles to climb the aquaria walls, escaping from the crabs’ reach, the apex of each snail was glued to a small tile with waterproof glue (Pattex® 100% Kleber-Colle). The tiles and all other prey items were randomly placed in the experimental units.
The experiment was carried out in eight separate runs in the same climate chamber where the crabs were housed, at a constant temperature of 15–17 °C and a day : night cycle of 12 h. Plastic tanks of 21 × 13.5 × 12 cm (L × W × H), covered with black film to prevent crabs from seeing each other, were randomly assigned a crab–prey treatment (one crab per tank). The tanks were filled with 1 litre of filtered seawater, provided with constant aeration and covered with a lid to hinder crabs from escaping. In total, the experiment was repeated eight times on different days, i.e. per run, two replicates per crab and prey treatment as well as two controls without crabs per prey treatment were carried out, resulting in a total of 16 replicates per crab species and prey treatment as well as 16 controls without crabs per prey treatment.
In the morning before each run, 12 crabs per species were separated and fed with crushed mussels to prevent any influence of starvation on food choice and consumption rate (Jubb et al., Reference Jubb, Hughes and p Rheinallt1983). During this feeding period, the species were held separately in plastic tanks provided with filtered seawater and aeration as well as stones and flowerpots as shelter. After 10.5 h of feeding, 10 crabs per species were individually put into the randomly assigned experimental tanks to acclimate overnight. To standardize hunger levels, no food was supplied during this acclimation period. In addition, two individuals of each species were kept separately to replace any potentially moulting crabs during the acclimation period. The replacement crabs were housed separately for each species in plastic tanks of the same type and size as the experimental tanks and provided with filtered seawater and aeration as well as stones and flowerpots for shelter. Similar to the crabs in the experimental tanks, no food was supplied before the experiment. After an acclimation period of 12 h, the experiment was started by introducing the previously prepared prey to each tank. If a crab had moulted during acclimation, it was removed from the tank and replaced by one of the replacement crabs. Crabs were given 24 h to feed, during which disturbances were kept to a minimum level. After this feeding period, the experiment was terminated by removing the crabs from the tanks. As before, sea lettuce leaves were dried with paper towels and their wet weight was measured to the nearest 0.1 mg. All other remaining prey items were counted and examined for possible damage.
Statistical analysis
All statistical analyses were carried out in R version 3.6.0 (26.4.2019) – ‘Planting of a Tree’ (R Core Team, 2019). For Bayesian models the brm function of the brms package (version 2.9.0) by Bürkner (Reference Bürkner2017, Reference Bürkner2018) was used. To analyse prey consumption in no-choice treatments, individual Bayesian models were fitted for each prey species. For the counts of remaining mussels and amphipods, models were fitted using a binomial distribution (link: logit), while the equivalent model for sea lettuce was fitted with a normal distribution. In all models, the amount of prey eaten was set as the response variable and crab treatment as the fixed effect. As we observed slight growth of sea lettuce during the experiment in the controls, we corrected the wet weight of the consumed sea lettuce of each crab treatment before the analysis (using the mean weight gain of all sea lettuce controls after the experiment).
As the experiment was carried out over several days, all model formulas contained a random part consisting of crab treatment as a random slope and day as the random intercept, allowing for day-specific differences in the effect of the crab treatments. As only a single periwinkle was consumed in all no-choice treatments, periwinkles were excluded from this analysis.
Prey consumption in choice treatments was analysed using a Bayesian multivariate mixed model with correlated random effects. For each prey species a separate brms formula was set up using a binomial distribution for mussels, amphipods and periwinkles and a Gaussian distribution for sea lettuce. As for the analysis of no-choice treatments, we corrected for the combined confounding effect of algal growth and consumption of algae by periwinkles or snails during the experiment (using the observed mean weight loss of sea lettuce in the control treatments).
To prevent numerical instabilities of the sampler, these corrected values for sea lettuce treatments were scaled to have a mean of zero and a standard deviation of one. In each model, the amount of prey eaten was used as the response variable and crab treatment as the fixed effect. The variables for individual crab numbers and day were used as random effects, allowing for varying correlated effects and accounting for day and individual specific preferences. Because in choice treatments all responses were correlated, the separate formulas were added together in a multivariate mixed model. As not a single snail was consumed during choice trials, we did not include them in the analysis.
For both intercepts and coefficients, weakly informative priors with a standard deviation of one were chosen to rule out possible unreasonable parameter values. Model fits were inspected visually by performing posterior predictive checks using the brms implemented pp_check function (Supplementary Appendix; Figures A1 and A2). Because brms does not provide a function similar to a post hoc test in frequentist analysis, parameters were compared via hypothesis testing. To investigate changes in the prey consumption between choice and no-choice treatments, the posterior samples of all models were compared. Raw data of all experiments can be found in Bleile & Thieltges (Reference Bleile and Thieltges2021).
Results
In no-choice treatments where crabs were only offered one prey species at a time, mussels (Mytilus edulis) were extensively consumed by all three crab species with no significant differences in the proportion consumed among crabs (Figure 1, Table 1). In all three crab species, mussel consumption was significantly higher than amphipod (Gammarus locusta) consumption (posterior probabilities >0.99; Figure 1, Table 2). Amphipod consumption differed among the three crab species, with Hemigrapsus takanoi consuming fewer amphipods than Carcinus maenas, whereas no significant difference was found between Hemigrapsus sanguineus and C. maenas, or between the two Hemigrapsus species (Figure 1, Table 1). Periwinkles (Littorina littorea) and sea lettuce (Ulva lactuca) were hardly consumed by the crabs (Figure 1, Table 1). In all no-choice treatments, only a single periwinkle was consumed by an individual of H. takanoi (Figure 1).
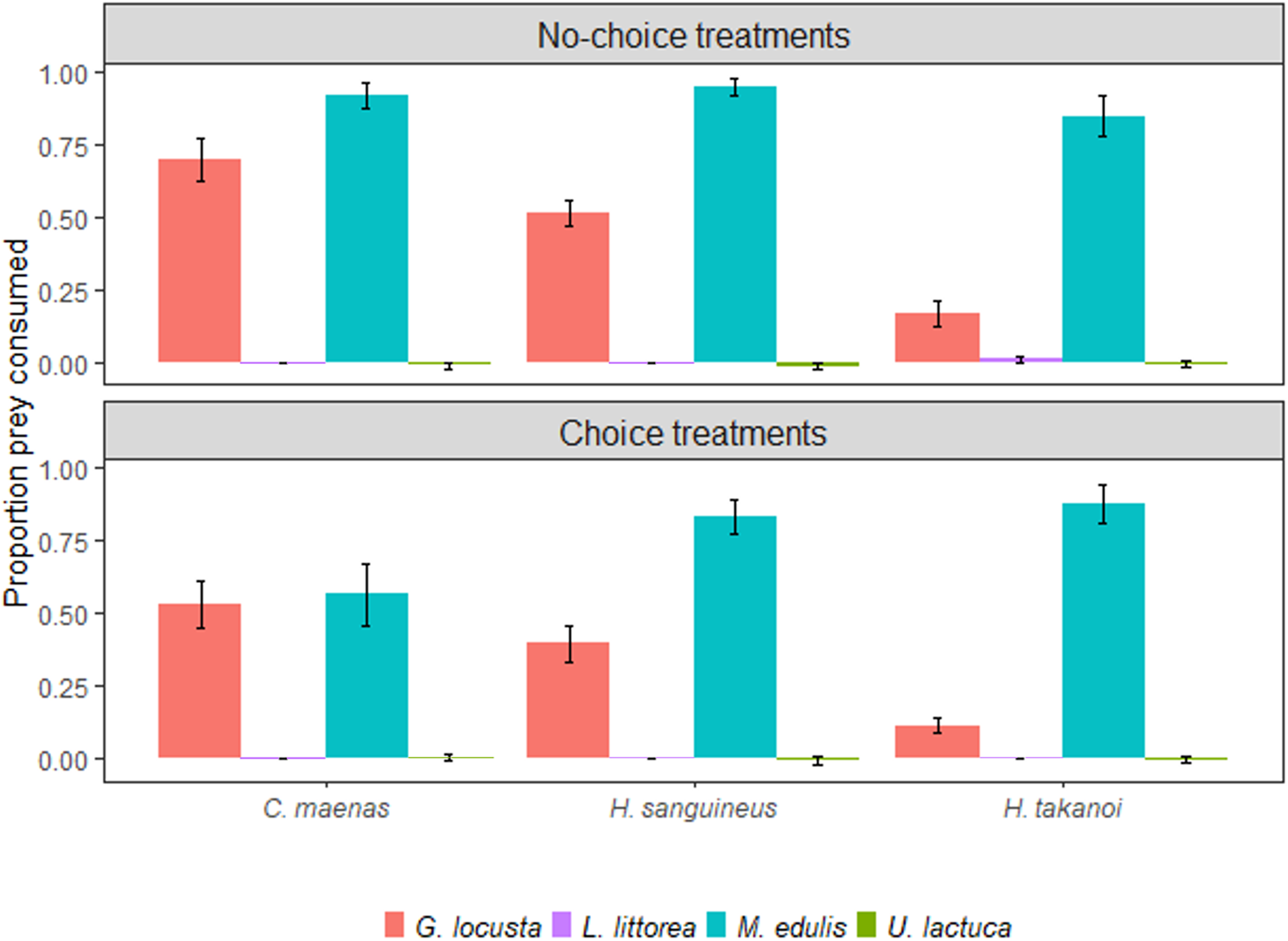
Fig. 1. Proportion of prey consumed (± SE) by the native shore crab Carcinus maenas and the invasive crabs Hemigrapsus sanguineus and Hemigrapsus takanoi in no-choice (top) and choice (bottom) treatments. Crabs were given 24 h to feed and offered amphipods (Gammarus locusta), periwinkles (Littorina littorea), mussels (Mytilus edulis) and/or sea lettuce (Ulva lactuca). N = 16 crabs per species and prey treatment.
Table 1. Posterior probabilities (P) and lower and upper confidence intervals (CI) for all tested hypotheses comparing prey consumption of native shore crabs Carcinus maenas (C) and the invasive crabs Hemigrapsus sanguineus (S) and Hemigrapsus takanoi (T) in (a) no-choice treatments when offered amphipods (Gammarus locusta), mussels (Mytilus edulis), periwinkles (Littorina littorea) and sea lettuce (Ulva lactuca) separately; and in (b) choice treatments when offered all prey items simultaneously.

As periwinkles were not consumed, they were excluded from the analyses. Significance level was set to 5%, hence posterior probabilities >0.95 or <0.05 indicate significant differences (denoted in bold).
Table 2. Posterior probabilities of all tested hypotheses comparing mussel (Mytilus edulis) vs amphipod (Gammarus locusta) consumption of native shore crabs Carcinus maenas and the invasive crabs Hemigrapsus sanguineus and Hemigrapsus takanoi in no-choice and choice treatments
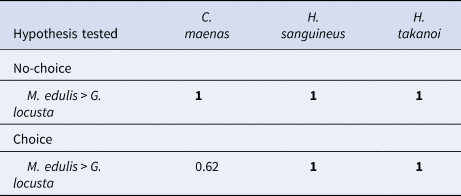
Significance level was set to 5%, hence posterior probabilities >0.95 or <0.05 indicate significant differences (denoted in bold).
In the choice treatments where crabs were simultaneously offered all four prey species, native C. maenas consumed significantly fewer mussels (M. edulis) than the invasive crabs H. takanoi and H. sanguineus, whereas the difference in mussel consumption between the two Hemigrapsus species was not significant (Figure 1, Table 1). For amphipods (G. locusta), results were similar to no-choice treatments and H. takanoi consumed significantly fewer amphipods than C. maenas and H. sanguineus, while there was no significant difference in amphipod consumption between C. maenas and H. sanguineus (Figure 1, Table 1). As in the no-choice treatments, sea lettuce (U. lactuca) and periwinkles (L. littorea) were barely consumed by all three crab species and no significant difference could be found between them (Figure 1, Table 1).
When comparing prey consumption between choice and no-choice treatments for the three crab species, the native C. maenas consumed significantly fewer mussels and amphipods when the prey community was more diverse (Figure 1, Table 3). In contrast, the invasive crabs H. sanguineus and H. takanoi did not show any significant changes in prey consumption when given a choice between several prey species (Figure 1, Table 3). Furthermore, only C. maenas showed a change in the ratio of mussel vs amphipod consumption depending on the prey diversity offered: while in no-choice treatments all three crab species consumed significantly more mussels than amphipods, C. maenas ate equal amounts of the two prey species when given a choice between them (Figure 1, Table 2). In contrast, the two Hemigrapsus species still consumed significantly more mussels than amphipods when offered both simultaneously (Figure 1, Table 2).
Table 3. Posterior probabilities for all tested hypotheses comparing prey consumption of native Carcinus maenas (C) and the invasive crabs Hemigrapsus sanguineus (S) and Hemigrapsus takanoi (T) between no-choice and choice treatments when offered mussels (Mytilus edulis), amphipods (Gammarus locusta), sea lettuce (Ulva lactuca) and/or periwinkles (Littorina littorea) either separately or at the same time

Significance level was set at 5%, hence posterior probabilities >0.95 or <0.05 indicate significant differences (denoted in bold). Periwinkles were rarely consumed and were excluded from the analysis (see text for details).
In the control treatments without crabs, all animals added (mussels, amphipods, periwinkles) survived, indicating that observed losses were indeed due to crab consumption. Algae in the no-choice experiments slightly increased due to growth (about 1%), while algae weight slightly decreased (about 4%) in the choice experiments, likely due to the combined effect of algae growth and algae consumption by amphipods and snails. The corrections applied to algae consumption by crabs based on the controls (see Methods) led to small negative consumption values in some cases (Figure 1) because consumption by algae-eating crabs was generally low and in some cases did not exceed algae growth.
Discussion
In both choice and no-choice treatments animal prey, and in particular mussels (Mytilus edulis), turned out to be the preferred prey of all three crab species, while algae were unanimously rejected by all of them. This confirms results from North America, where European shore crabs (Carcinus maenas) and Asian shore crabs (Hemigrapsus sanguineus) showed a similar preference for animal prey over algae (Elner, Reference Elner1981; Brousseau & Baglivo, Reference Brousseau and Baglivo2005; Griffen, Reference Griffen, Galil, Clark and Carlton2011). However, our experiments could not confirm suggestions that invasive Asian shore crabs may have a more herbivorous diet than European shore crabs, as based on results from gut content analyses of crabs collected in the field in North America (Lohrer et al., Reference Lohrer, Whitlatch, Wada and Fukui2000; Griffen, Reference Griffen, Galil, Clark and Carlton2011; Griffen et al., Reference Griffen, Altman, Bess, Hurley and Penfield2012) and trophic marker analyses of lipids in crabs collected in Europe (Jungblut et al., Reference Jungblut, McCarthy, Boos, Saborowski and Hagen2018). This discrepancy between results from laboratory experiments and field samples may point to more complex feeding behaviours of the invasive crabs in the field. Here, crabs will most likely have to switch to more opportunistic feeding, depending on the actual availability of prey types in their respective habitat. In addition, when hungry, crabs may switch to a wider diet than in experimental settings as documented in laboratory experiments where Asian shore crabs consumed more algae when starved for several days (Brousseau & Baglivo, Reference Brousseau and Baglivo2005). Hence, when resources are limited in the field, invasive crabs might show a higher level of herbivory. Given the paucity of feeding preference data of the invasive crabs from European locations, more research is needed that links findings from laboratory experimentation with field sampling in different habitats (e.g. using gut content analyses, stable isotope analyses or trophic markers in lipids) to identify levels of herbivory under different conditions.
While it has already been shown that mussels serve as an important prey for the two invasive crab species in Europe (Bouwmeester et al., Reference Bouwmeester, Waser, van der Meer and Thieltges2020; Nour et al., Reference Nour, Stumpp, Morón Lugo, Barboza and Pansch2020), our experiments indicate that they feed on mobile amphipods as well. This corroborates findings from North America where H. sanguineus has also been shown to consume amphipods (Griffen, Reference Griffen, Galil, Clark and Carlton2011; Blasi & O'Connor, Reference Blasi and O'Connor2016) and it suggests that the prey spectrum of H. sanguineus may be similar at invaded locations on both sides of the Atlantic. However, both invasive crabs consumed fewer amphipods than mussels. This may point to a preference for mussels over amphipods or, alternatively, to some difficulties in catching motile prey. In contrast, the native crabs consumed a higher proportion of amphipods than the invasive crabs and they fed at similar rates on mussels and amphipods in choice treatments. We can only speculate about the mechanisms underlying these differences in feeding patterns, but the different claw morphologies of native and invasive crabs provide a possible explanation. While the invasive crabs have claws that are relatively short and stunted, the claws of the native crabs are longer and more slender, making it presumably easier for them to catch motile prey. It is also noteworthy that the invasive H. takanoi consumed significantly fewer amphipods than its sibling species H. sanguineus. Hence, our assumption that the diets of the two Hemigrapsus species will be similar to each other, resulting from their close relation and origin, is only partly true. Again, differences in claw morphology might play a role in explaining the observed differences but more research will be needed in this respect.
Although the three crab species preferred animal prey, they all rejected the periwinkles offered to them in the experiments. For H. sanguineus, an avoidance of L. littorea has also been observed in studies from North America (Bourdeau & O'Connor, Reference Bourdeau and O'Connor2003). This avoidance is likely explained by the relatively more robust shell of the snails compared with mussels, increasing the risk of damaging the crab's claws when attempting to open them. Since the crabs were not extensively starved before the experiment, their hunger levels were probably not high enough to take this risk. It is also possible that the method of immobilising snails by gluing them to small tiles (to prevent them from climbing out of the experimental units) may have affected the crabs’ prey choice. However, larger native crabs are able to consume snails offered in this way (pers. obs.), so the avoidance is likely to be linked to shell strength.
While the two invasive crab species showed a similar prey preference and consumption rates in choice and no-choice treatments, the native crabs showed a significant change in diet when the prey community was more diverse. When given a choice, native crabs no longer preferred mussels over amphipods and they generally consumed significantly fewer mussels and amphipods compared with no-choice treatments. The lack of preference in choice treatments might indicate that native crabs broaden their diet when multiple prey species are available. However, they did not increase their general consumption but instead reduced their overall consumption. This reduced consumption might result from mussels and amphipods hiding in the algae or under the tiles to which the snails were attached, making them unavailable for the crabs. Such a mediating role of structural predation refuges on crab consumption rates is well known (Waser et al., Reference Waser, Splinter and van der Meer2015). Interestingly, both invasive crabs did not show a significant change in mussel or amphipod consumption between no-choice and choice treatments and both still preferred mussels over amphipods. It remains to be studied whether invasive crabs are better at tracing hidden prey items than native crabs or whether the reduced consumption of native crabs in choice compared with no-choice treatments results from other mechanisms.
The observed prey preferences of the three crab species have two important implications. First of all, the strong preference for mussels in all three species suggests that this could result in resource competition between native and invasive crabs. Previous experiments on mussel consumption by crabs from European locations also indicated that the preferred prey size ranges overlap between the invasive and native crabs (Bouwmeester et al., Reference Bouwmeester, Waser, van der Meer and Thieltges2020). Hence, it is highly likely that crabs co-occurring in specific habitats will compete for mussels. In contrast, competition for amphipods can be expected to be lower between invasive and native crabs and will most likely differ between the two invasive crabs as well. However, the actual magnitude of competition in the field will likely be mediated by prey availability and crab densities as indicated by studies of North American densities (Griffen et al., Reference Griffen, Guy and Buck2008). In addition, it is also possible that ontogenetic shifts in diet choice may occur in the three crab species so that competition effects in the field will also depend on the relative densities of different age classes.
The second implication of the observed prey preferences is that the strong preference for mussels by the invasive crabs likely increases the predation pressure on native mussels at localities invaded by these crabs. At many locations in the Wadden Sea (as elsewhere in the invaded range), the invasive crabs are much more abundant than the native crabs (Van den Brink et al., Reference van den Brink, Wijnhoven and McLay2012; Landschoff et al., Reference Landschoff, Lackschewitz, Kesy and Reise2013; Jungblut et al., Reference Jungblut, Beermann, Boos, Saborowski and Hagen2017; Van den Brink & Hutting, Reference van den Brink and Hutting2017; Geburzi et al., Reference Geburzi, Brandis and Buschbaum2018). Hence, any additional predation by the invasive crabs is likely increasing the predation pressure on native mussels, with potential effects on local mussel population sizes. In contrast, native amphipod populations may be less affected by invasive crabs, particularly at locations invaded by H. takanoi, given the lower preference observed in our experiments. However, further research into the population-level consequences for native prey species and communities is needed.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315421000655
Acknowledgements
Special thanks go to Mark Bouwmeester and Nicole Bleile for their great help with field- and laboratory work. We also thank Annika Cornelius and Christian Buschbaum for comments on a draft version of the manuscript.








