No CrossRef data available.
Article contents
Patterns of Organization of Cerebellum and Spinal Cord of the Red-Tail Shark (Epalzeorhynchos bicolor): Histological, Morphometrical, and Immunohistochemical Studies
Published online by Cambridge University Press: 14 October 2020
Abstract
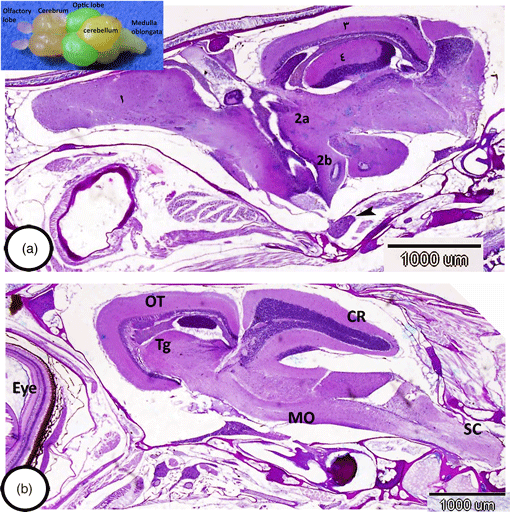
Teleosts exhibit enormous heterogeneity in brain morphology, especially in the patterns of the organization of cerebellum. The cerebellum of a red-tail shark that we analyzed was well-developed and included three main divisions: the valvula cerebelli, the corpus cerebelli, and the vestibulolateral lobe. Characteristically, the cerebellar cortex contained three well-distinct layers: an outer molecular, intermediate ganglionic, and inner granular layer. The ganglionic layer possessed irregularly arranged Purkinje cells and eurydendroid cells that extended their processes into the molecular layer. Both Purkinje cells and eurydendroid cells showed immunoreactivity for iNOS2. Moreover, astrocytes in the cerebellum showed the expression of glial fibrillary acidic protein. The most striking observation in the cerebellum of shark was the lack of deep cerebellar nuclei and a well-identified white matter. On the other hand, the gray substance in the spinal cord displays a characteristic pattern in its organization, in which the dorsal horns lie quite close together, giving the gray substance the shape of an inverted Y and possessing large neurons. Notably, the white matter possessed myelinated nerve fibers. The current study provides the first report on the organization of layers and neurons in the cerebellum and spinal cord of red-tail shark. This research will contribute to the neuroanatomy and evolutionary studies of the brain of Cyprinidae.
- Type
- Micrographia
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of the Microscopy Society of America




