Article contents
Goldhillite, Cu5Zn(AsO4)2(OH)6⋅H2O, a new mineral species, and redefinition of philipsburgite, Cu5Zn[(AsO4)(PO4)](OH)6⋅H2O, as an As–P ordered species
Published online by Cambridge University Press: 13 May 2022
Abstract
Philipsburgite has been redefined as the intermediate member of the goldhillite–philipsburgite–kipushite isomorphous series with the ideal formula Cu5Zn[(AsO4)(PO4)](OH)6⋅H2O due to the site-selective As–P substitution. The new mineral goldhillite, ideally Cu5Zn(AsO4)2(OH)6⋅H2O [or Cu5Zn(AsO4)(AsO4)(OH)6⋅H2O], is the arsenate end-member of this series. Goldhillite occurs on fracture surfaces in a rock comprised mostly of quartz with iron hydroxides in association with mixite, cornwallite and conichalcite. Goldhillite forms transparent, bright emerald-green, tabular crystals with vitreous lustre, flattened on {100}, up to 1 mm across and in rosettes up to 1.5 mm. The mineral is brittle with uneven fracture and perfect cleavage on {100}; the Mohs hardness is 3.5. The calculated density for the holotype is 4.199 g cm–3. The Raman spectrum is consistent with the presence of H2O-molecules, OH-groups, AsO4 tetrahedra and traces of PO4. Electron microprobe analyses of goldhillite (H2O content based on the crystal structure) provided: CuO 48.91, ZnO 13.18, As2O5 26.06, P2O5 3.25, H2O 8.97, total 100.37 wt.%. The empirical formula for goldhillite based on O = 15 apfu is (Cu4.69Zn1.23)Σ5.92(As0.86P0.18O4)2(OH)5.61⋅H2O. The crystal structures of goldhillite and philipsburgite were determined using single-crystal X-ray diffraction data and refined to R1 = 0.054 (for 2365 I > 2σI reflections) and 0.052 (for 2308 I > 2σI reflections), respectively. Goldhillite is monoclinic, P21/c, a = 12.3573(5), b = 9.2325(3), c = 10.7163(4) Å, β = 97.346(4)°, V = 1212.59(8) Å3 and Z = 4. Philipsburgite is monoclinic, P21/c, a = 12.3095(9), b = 9.2276(3), c = 10.7195(3) Å, β = 97.137(7)°, V = 1208.16(10) Å3 and Z = 4. The strongest lines of the powder X-ray diffraction pattern of goldhillite [d, Å (I, %)(hkl)] are: 4.09 (28)(300), 3.41 (23)(12$\bar{2}$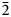 , 221, 311), 2.57 (100)(132, 11$\bar{4}$
, 221, 311), 2.57 (100)(132, 11$\bar{4}$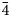 , 20$\bar{4}$
, 20$\bar{4}$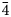 ), 2.17 (18)(42$\bar{3}$
), 2.17 (18)(42$\bar{3}$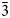 , 332), 1.95 (22)(432) and 1.54 (20)(13$\bar{6}$
, 332), 1.95 (22)(432) and 1.54 (20)(13$\bar{6}$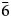 , 060). Goldhillite is named after its type locality, the Gold Hill mine, Tooele County, Utah, USA.
, 060). Goldhillite is named after its type locality, the Gold Hill mine, Tooele County, Utah, USA.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Juraj Majzlan
References
- 2
- Cited by




