Article contents
Hloušekite, (Ni,Co)Cu4(AsO4)2(AsO3OH)2(H2O)9, a new member of the lindackerite supergroup from Jáchymov, Czech Republic
Published online by Cambridge University Press: 05 July 2018
Abstract
Hloušekite, (Ni,Co)Cu4(AsO4)2(AsO3OH)2(H2O)9, is a new supergene arsenate mineral from the Geister vein (Rovnost mine), Jáchymov (St Joachimsthal), Western Bohemia, Czech Republic. It was found along with veselovský ite, pradetite, lavendulan, arsenolite, babánekite and gypsum on the surface of strongly altered ore fragments containing dominant tennantite and chalcopyrite. Hloušekite forms thin, lath-like crystals, locally elongated reaching up to 3 mm across. It is transparent, has a pale green colour with vitreous lustre, has a greyish-white streak and it is very brittle with an uneven fracture. It does not fluoresce under shortwave or longwave ultraviolet radiation. Cleavage on {010} is perfect; the Mohs hardness is 2–3. The calculated density is 3.295 g cm–3. Hloušekite is optically biaxial with α’ = 1.653(2) and γ’ = 1.73. The estimated optical orientation is γ’ vs. elongation (c) = 14(1)°. In larger grains it is weakly to moderately pleochroic (α = colourless, γ = pale green to green). Hloušekite is triclinic, space group P , a = 6.4010(6), b = 8.0041(6), c = 10.3969(14) Å , α = 85.824(8), β = 79.873(9), γ = 84.655(7)° and V = 521.23(10) Å3, with Z = 1, a:b:c = 0.800:1:1.299. The eight strongest lines in the powder X-ray diffraction (XRD) pattern [d in Å (I)(hkl)] are 10.211(100)(001), 7.974(9)(010), 3.984(6)(020), 3.656(5)(1
, a = 6.4010(6), b = 8.0041(6), c = 10.3969(14) Å , α = 85.824(8), β = 79.873(9), γ = 84.655(7)° and V = 521.23(10) Å3, with Z = 1, a:b:c = 0.800:1:1.299. The eight strongest lines in the powder X-ray diffraction (XRD) pattern [d in Å (I)(hkl)] are 10.211(100)(001), 7.974(9)(010), 3.984(6)(020), 3.656(5)(1 2), 3.631(5)(0
2), 3.631(5)(0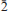 1), 3.241(5)(022), 3.145(5)(200) and 3.006(5)(210). Chemical analysis by electron microprobe yielded MgO0.20, FeO0.10, NiO 5.79, CoO1.80, CuO29.53, ZnO 0.66, Al2O3 0.14, P2O5 0.11, As2O5 45.01, H2O 17.71 (calc.), for a total of 101.05 wt.%. The resulting empirical formula, calculated by stoichiometry (9H2O + 2OH), obtained from the crystal structure, is (Ni0.79Co0.25)Σ1.04(Cu3.78Zn0.08Mg0.05Al0.03Fe0.01)Σ3.95 (AsO4 )1.98(PO4 )0.02(AsO3OH)2.00(H2O)9.00 . The ideal endmember formula , NiCu4(AsO4)2(AsO3OH)2(H2O)9.00, requires NiO7.23, CuO30.81, As2O5 44.51, H2O17.45, total 100.00 wt.%. The crystal structure of hloušekite was solved by charge flipping from single-crystal XRD data and refined to R1 = 0.0599 for 1441 reflections with [Iobs > 3σ(I)]. Hloušekite is a new member of the lindackerite group (also including lindackerite, pradetite and veselovský ite) of the lindackerite supergroup. The ondrušite group of the lindackerite supergroup includes ondrušite, chudobaite, geigerite and klajite. The establishment of these two groups reflects the difference between the crystal structures of their members, mainly in the coordination environment of the Me cations.
1), 3.241(5)(022), 3.145(5)(200) and 3.006(5)(210). Chemical analysis by electron microprobe yielded MgO0.20, FeO0.10, NiO 5.79, CoO1.80, CuO29.53, ZnO 0.66, Al2O3 0.14, P2O5 0.11, As2O5 45.01, H2O 17.71 (calc.), for a total of 101.05 wt.%. The resulting empirical formula, calculated by stoichiometry (9H2O + 2OH), obtained from the crystal structure, is (Ni0.79Co0.25)Σ1.04(Cu3.78Zn0.08Mg0.05Al0.03Fe0.01)Σ3.95 (AsO4 )1.98(PO4 )0.02(AsO3OH)2.00(H2O)9.00 . The ideal endmember formula , NiCu4(AsO4)2(AsO3OH)2(H2O)9.00, requires NiO7.23, CuO30.81, As2O5 44.51, H2O17.45, total 100.00 wt.%. The crystal structure of hloušekite was solved by charge flipping from single-crystal XRD data and refined to R1 = 0.0599 for 1441 reflections with [Iobs > 3σ(I)]. Hloušekite is a new member of the lindackerite group (also including lindackerite, pradetite and veselovský ite) of the lindackerite supergroup. The ondrušite group of the lindackerite supergroup includes ondrušite, chudobaite, geigerite and klajite. The establishment of these two groups reflects the difference between the crystal structures of their members, mainly in the coordination environment of the Me cations.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2014
References
- 6
- Cited by




