Article contents
Incorporation of Fe3+ in phase-X, A2–xM2Si2O7Hx, a potential high-pressure K-rich hydrous silicate in the mantle
Published online by Cambridge University Press: 05 July 2018
Abstract
Phase-X, a potential sink for K in the mantle, is a synthetic hydrous K-rich silicate formed by the breakdown of K-amphibole at high pressure. It has the general formula A2–xM2Si2O7Hx where A = K, Na, Ca, □ (vacancy), and M = Mg, Al, or Cr. No other isomorphic substitutions, either for the A or the M site, were reported for such a synthetic compound. Here we report the crystal structure and chemical composition of a crystal of phase-X containinglarg e amounts of trivalent Fe. This crystal was synthesized in the model system garnet lherzolite–K2CO3 at lower pressure (P = 7 GPa) and higher temperature (T = 1450–1650°C) with respect to the stability range reported in the literature (i.e. P = 9–17 GPa and T = 1150–1400°C). Quantitative analysis led to the following formula: 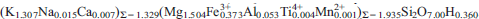 . The lattice parameters (hexagonal setting) are: a = 5.005(1), c = 13.148(2) Å, V = 285.23(9) Å3, and Z = 2. The structure was refined in space group P63cm to R = 5.06% using199 independent reflections and consists of MO6 octahedra layers stacked alongthe c axis and linked together by Si2O7 groups. The Si2O7 groups form pillars in the layer that contains A atoms in the cavities between the pillars. The Raman spectrum in the OH-stretching region indicates the existence of two different OH environments. However, the position of H could not be determined. The substitution of Fe3+ for Mg shortens octahedral bond distances. In addition, the entry of Fe3+ in M induces geometrical changes to the adjacent A site. The crystal-chemical characteristics are compared with published data on synthetic Fe-free phase-X.
. The lattice parameters (hexagonal setting) are: a = 5.005(1), c = 13.148(2) Å, V = 285.23(9) Å3, and Z = 2. The structure was refined in space group P63cm to R = 5.06% using199 independent reflections and consists of MO6 octahedra layers stacked alongthe c axis and linked together by Si2O7 groups. The Si2O7 groups form pillars in the layer that contains A atoms in the cavities between the pillars. The Raman spectrum in the OH-stretching region indicates the existence of two different OH environments. However, the position of H could not be determined. The substitution of Fe3+ for Mg shortens octahedral bond distances. In addition, the entry of Fe3+ in M induces geometrical changes to the adjacent A site. The crystal-chemical characteristics are compared with published data on synthetic Fe-free phase-X.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2007
References
- 7
- Cited by


