Introduction
The mineral gadolinite has a special place in the history of science, and specifically so in chemistry and mineralogy. It was the study of what was then called “ytterbite” from the Ytterby pegmatite north of Stockholm, Sweden, by the Finnish-Swedish chemist Johan Gadolin (1760–1852), that led to the first discovery of a rare-earth element (REE) in the form of “yttria” (Gadolin, Reference Gadolin1794). Gadolin's yttria was later proven to be a mixture of REEs, dominated by yttrium, while the “ytterbite” was finally named gadolinite (Ekeberg, Reference Ekeberg1802), now gadolinite-(Y). Presently, the REEs are very high on the European Union's lists of critical metals (http://europa.eu/rapid/press-release_IP-14-599_en.htm) and neodymium is the most in demand. Systematic research towards the understanding of REE ore-forming processes, a better knowledge of the distribution of these metals among the individual ore minerals, and the origin of these metals is of increasing interest.
The newly-defined gadolinite supergroup (Bačík et al., Reference Bačík, Miyawaki, Atencio, Cámara and Fridrichová2017) includes silicates, phosphates and arsenates with the general formula A 2MQT 2O 8φ2, where the individual structural sites are occupied as follows A: Ca, REE (Y + lanthanoids) > U, Th, Pb, Mn2+ and Bi; M: Fe2+, □ (vacancy) > Mg, Mn2+, Zn, Cu, Al; Q: Be, B > Li; T: Si > P, As, B, Be, S; φ: O, OH > F (Bačík et al., Reference Bačík, Miyawaki, Atencio, Cámara and Fridrichová2017). Based on the T-site occupancy, the gadolinite supergroup is divided into the gadolinite [Si4+ > (P5+ + As5+)] and herderite [(P5+ + As5+) > Si4+] groups. Moreover, there are two subgroups within the gadolinite group, of which the gadolinite subgroup is dominated by REE 3+ cations, and the datolite subgroup is dominated by divalent cations, in the A site. Accordingly, the Q-site is occupied predominantly by Be2+ in the gadolinite subgroup and by B3+ in the datolite subgroup. The herderite group is also subdivided into the herderite and drugmanite subgroups (cf. Table 1).
Table 1. Minerals of the gadolinite supergroup and their structural formulae (Bačík et al., Reference Bačík, Miyawaki, Atencio, Cámara and Fridrichová2017).
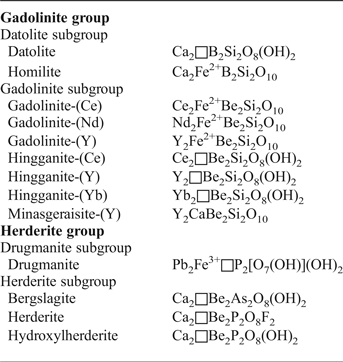
Gadolinite-subgroup minerals (particularly gadolinite and hingganite) typically occur in rather metaluminous, fractionated granitic pegmatites with a NYF (Niobium-Yttrium-Fluorine; in the sense of Černý, Reference Černý1991) signature (e.g. Bjørlykke, Reference Bjørlykke1935; Brotzen, Reference Brotzen1959; Haynes, Reference Haynes1965; Vorma et al., Reference Vorma, Ojanperä, Hoffren, Siivola and Löfgren1966; Nilssen, Reference Nilssen1973; Bergstøl and Juve, Reference Bergstøl and Juve1988; Smeds, Reference Smeds1990; Kjellman et al., Reference Kjellman, Černý and Smeds1999; Pezzotta et al., Reference Pezzotta, Diella and Guastoni1999; Miyawaki et al., Reference Miyawaki, Matsubara, Yokoyama and Okamoto2007; Škoda et al., Reference Škoda, Cempírek, Filip, Novák, Veselovský and Čtvrtlík2012, Reference Škoda, Plášil, Jonsson, Čopjaková, Langhof and Vašinová Galiová2015; Pieczka et al., Reference Pieczka, Hawthorne, Cooper, Szełęg, Szuszkiewicz, Turniak, Nejbert and Ilnicki2015), alkaline rocks (e.g. Segalstad and Larsen, Reference Segalstad and Larsen1978; Cámara et al., Reference Cámara, Oberti, Ottolini, Della Ventura and Bellatreccia2008; Pekov et al., Reference Pekov, Pasero, Yaskovskaya, Chukanov, Pushcharovsky, Merlino, Zubkova, Kononkova, Menshikov and Zadov2007; Lyalina et al., Reference Lyalina, Selivanova, Savchenko, Zozulya and Kadyrova2014), REE-rich Alpine-type hydrothermal mineralizations (e.g. Demartin et al., Reference Demartin, Pilati, Diella, Gentile and Gramaccioli1993; Bonazzi et al., Reference Bonazzi, Bindi and Parodi2003; Pršek et al., Reference Pršek, Ondrejka, Bačík, Budzyń and Uher2010), and some iron-oxide skarn deposits (e.g. Geijer, Reference Geijer1961; Holtstam and Andersson, Reference Holtstam and Andersson2007).
The members of the gadolinite subgroup with a magmatic origin are commonly metamict due to significant amounts of actinides substituting for REE, which complicates their structural investigation. Well-crystalline samples are scarce and usually of a hydrothermal origin (Demartin et al., Reference Demartin, Pilati, Diella, Gentile and Gramaccioli1993).
A neodymium-dominant analogue of gadolinite-(Y) or gadolinite-(Ce) was suggested by Holtstam and Andersson (Reference Holtstam and Andersson2007) from Bastnäs-type skarn deposits (Bergslagen ore province, south-central Sweden), but due to the lack of a sufficient amount of material, they did not provide any data apart from the chemical microanalyses. This paper provides a full characterization, including chemical composition, structural, physical and optical properties of the new mineral gadolinite-(Nd), which was approved recently by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA), under the number 2016-013. The name is given in analogy with the other members of the gadolinite subgroup (Bačík et al., Reference Bačík, Miyawaki, Atencio, Cámara and Fridrichová2017), as the neodymium-dominant species. Type material is deposited under catalogue number B 11298 at the Moravian Museum, Brno, Czech Republic.
Geological setting and the type locality
Gadolinite-(Nd) was found in samples from the Malmkärra, Johanna and Nya Bastnäs mines, all belonging to the Fe-REE ‘Bastnäs-type’ skarn deposits in the Bergslagen ore province (Geijer, Reference Geijer1961; Holtstam and Andersson, Reference Holtstam and Andersson2007; Jonsson et al., Reference Jonsson, Högdahl, Sahlström, Nysten and Sadeghi2014; Holtstam et al., Reference Holtstam, Andersson, Broman and Mansfeld2014). The present mineral characterization was only undertaken on material from the Malmkärra mine (60°4′N, 15°51′E), ~3.5 km WSW of Norberg due to the very small size of gadolinite-(Nd) crystals (<30 µm) at the other localities.
The Bastnäs-type deposits occur in the northwestern part of the Bergslagen ore province, south central Sweden, in the so-called ‘REE-line’ (Jonsson and Högdahl, Reference Jonsson and Högdahl2013; Goodenough et al., Reference Goodenough, Schilling, Jonsson, Kalvig, Charles, Tuduri, Deady, Sadeghi, Schiellerup, Müller, Bertrand, Arvanitidis, Eliopolous, Shaw, Thrane and Keulen2016). This is an over 100 km long, north-east-trending, narrow belt within Svecofennian (1.91–1.88 Ga), mainly felsic metavolcanic rocks with discontinuous, interlayered carbonate (marble) horizons. The volcanic sequences were intensively metasomatized at the synvolcanic stage, and much of the carbonates have been replaced by skarn, hosting the localized Fe-REE mineralizations. The skarn-hosted Fe-oxide-REE-polymetallic mineralization at Bastnäs, in the central part of the REE-line, has been dated from the Re-Os contents of molybdenite to give ages between 1.90 and 1.84 Ga. The mineralization is suggested to be the products of reactions between carbonate interlayers with Si, F, Cl, S, CO2 and REE-bearing, high-temperature fluids of a magmatic origin (Holtstam et al., Reference Holtstam, Andersson, Broman and Mansfeld2014; Sahlström et al., Reference Sahlström, Jonsson, Högdahl, Harris, Troll and Jolis2015). The term ‘Bastnäs-type deposits’ was introduced by Geijer (Reference Geijer1961) to characterize a group of similar, Fe + REE ± Cu ± Mo ± Bi ± Au mineralized skarns that supposedly have a common origin to the Nya Bastnäs deposit. Based mainly on differences in mineral assemblages, Holtstam and Andersson (Reference Holtstam and Andersson2007) divided them into two subtypes: deposits of subtype 1 are essentially only enriched in light-rare-earth elements (LREE), whereas those of subtype 2 are enriched in both light and heavy-rare-earth elements (LREE and HREE, respectively).
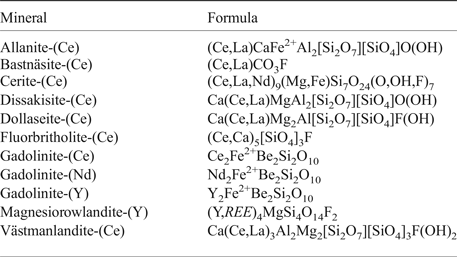
The Malmkärra deposit (subtype 2) is hosted by a partly dolomitic marble that forms a conformable interlayer within the surrounding, typically extensively altered metavolcanic rocks. The skarn ore body, formed through reactions and replacement of parts of the carbonate interlayer, occurs along the contact with the more extensively altered metavolcanic rocks. Magnetite is the sole ore mineral that was mined, and tremolitic amphibole, humite-group minerals, commonly serpentine-altered, biotite and phlogopite make up the main skarn silicates; sulfide minerals (pyrite, chalcopyrite and molybdenite) are locally present as well (Geijer, Reference Geijer1936). Allanites sensu lato represent the most widespread REE minerals scattered in the skarn, as well as in the iron ore. During mining, a ~0.5 m thick zone of massive REE silicates [dominated by fluorbritholite-(Ce), västmanlandite-(Ce) and dollaseite-(Ce)] was encountered in the deeper levels of the mine, along the contact between the marble and the skarn iron ore (Geijer, Reference Geijer1936; Andersson, Reference Andersson2004). The REE minerals reported from the Malmkärra mine are summarized in Table 2.
Table 2. List of REE-minerals from the Malmkärra mine, after Holtstam and Andersson (2007), Holtstam et al. (2005).
The Malmkärra iron mine has a long history. The oldest preserved record is from 1664, with major mining operations taking place from 1885 up to its final closure in 1936 (Geijer and Magnusson, Reference Geijer and Magnusson1944; Andersson, Reference Andersson2004). Malmkärra is also the type locality of västmanlandite-(Ce), see Holtstam et al. (Reference Holtstam, Kolitsch and Andersson2005).
Results
Mineral association, appearance and physical properties
The skarn rock sample (~10 cm × 10 cm × 5 cm) containing gadolinite-(Nd) was found on the dumps of the Malmkärra mine. Gadolinite-(Nd) forms anhedral grains up to 150 µm in size, occurring as aggregates associated with tremolite, dollaseite-(Ce), västmanlandite-(Ce), fluorbritholite-(Ce) and bastnäsite-(Ce) (Fig. 1). Gadolinite-(Nd) is transparent and, under the optical microscope in thin section, it is pale olive green, while larger fragments are olive green. It has a white streak and a vitreous to adamantine lustre. No visible fluorescence under shortwave or longwave ultraviolet light was observed. The Mohs hardness is between 6.5 and 7. Gadolinite-(Nd) is brittle with a conchoidal fracture; no cleavage or parting was observed. The density (D calc) is 4.86 g cm–3, and was calculated using the empirical formula and unit-cell parameters obtained from the single-crystal X-ray diffraction (XRD) data. A density measurement could not be performed directly due to the lack of suitable material. Optically, gadolinite-(Nd) is weakly pleochroic in shades of olive green, biaxial (–), nα = 1.78(1), nβ(calc.) = 1.80, nγ = 1.81(1) measured in white light. The poor optical quality of the measured fragments did not allow more precise determination of the refractive indices. The angle of the optical axes, 2V(meas.): 62(3)° was measured using extinction by means of a spindle stage and computed by the EXCALIBR II software. Dispersion is strong (r < v).
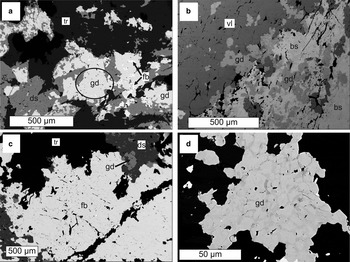
Fig. 1. Back-scattered electron images of gadolinite-(Nd) and associated REE minerals from (a–c) Malmkärra and (d) Johannagruvan. gd-gadolinite-(Nd), ds-dollaseite-(Ce), fb-fluorbritholite-(Ce), tr-tremolite, vl-västmanlandite-(Ce), bs-bastnäsite-(Ce). The aggregate from which the crystals for single-crystal X-ray diffraction and optical study were extracted is indicated by an ellipse. The zoning of the gadolinite-(Nd) from Johannagruvan (d) reflects Y-lanthanoids variability; the Y-rich zones are darker.
Composition
The compositions of gadolinite-(Nd) and associated minerals were determined by means of electron-probe microanalysis (EPMA) with a CAMECA SX100 in wavelength-dispersive mode at an accelerating voltage of 15 kV, a beam current 20 nA and a beam diameter of 5 µm. The standards, X-ray lines and monochromators used for analysis are listed in Table 2. Aluminium, Pb, Tm, Yb and Lu were also analysed for, but their contents were below the detection limit. The peak counting times varied from 10 s for the main elements to 60 s for the minor elements and high and low energy background was counted for ½ of the peak counting time of the relevant analytical line. The raw data were processed using the X-phi matrix correction routine (Merlet, Reference Merlet1994). Based on the counting statistics, the measurement error expressed as 2σ is approximately <1 rel.% for concentrations of ~20 wt.% and ~8 rel.% for concentrations of ~1=wt.%.
The contents of Be, B and other trace elements below the detection limit of the EPMA were sought for by means of an Agilent 7500ce quadrupole Inductively Coupled Plasma Mass Spectrometer (ICP-MS; Agilent 7500ce, Santa Clara, CA, USA) with an attached UP 213 laser ablation (LA) system (New Wave Research, Inc., Fremont, CA, USA). The LA-ICP-MS system consists of a nanosecond laser Nd:YAG operating at 213 nm (pulse duration of 4.2 ns) and a SuperCell ablation chamber. Ablated material was transported from the sample chamber using helium carrier gas (1 L min–1) and mixed with argon (0.6 L min–1) prior to the torch. Potential polyatomic interferences were minimized by a collision reaction cell in He mode (1 mL min–1). The contents of major to trace elements were determined after laser ablation of individual spots at the following conditions: diameter 55 µm, strength of laser beam 4 J cm–2, frequency 10 Hz and spot ablation time 30 s. The contents of elements of interest were determined using SRM NIST 610 and 28Si for internal calibration and the calculations were performed via peak area of isotopes.
The empirical formula has been calculated on the basis of 2 atoms per formula unit of Si and the amount of OH has been charge balanced to keep the empirical formula electroneutral.
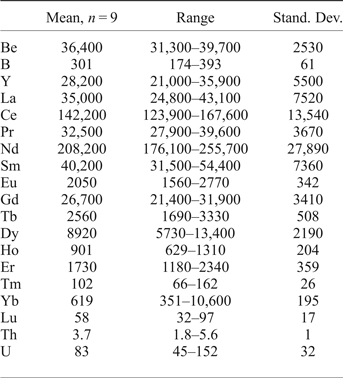
The composition of gadolinite-(Nd) was examined from three thin sections prepared from a single piece of the massive REE-silicate-rich sample. The gadolinite shows quite variable chemical composition, particularly in the REE pattern and the Y/(REE) ratio, but all analyses (n = 11) are Nd-dominant and correspond to gadolinite-(Nd), see Table 3. The calcium content is low (0.14–0.24 wt.% CaO). Holtstam and Andersson (Reference Holtstam and Andersson2007) performed Mössbauer spectroscopy on the gadolinite-(Ce/Y) from Malmkärra, which revealed the presence of exclusively divalent Fe. Therefore, all Fe is assumed as divalent in gadolinite-(Nd). Apart from Fe, the M site is occupied by Mg (0.51–0.66 wt.% MgO) and Mn (0.10–0.14 wt.% MnO). All these cations do not fill the M site completely, thus a minor vacancy (0.093–0.143 □ pfu) is present. Seven grains, previously analysed by EPMA, were subsequently analysed by LA-ICP-MS to determine the Be and B content as well as the whole suite of the REEs and some trace elements (Table 4). A chondrite-normalized REE pattern (using a combination of EPMA and LA-ICP-MS data) shows the maxima at Nd and depletion of the lightest and heavy REE as well as a moderate Eu anomaly (EuN*/EuN = 0.16), see Fig. 2. The content of BeO obtained from the LA-ICP-MS analysis ranges from 8.69 to 11.02 wt.% with a mean of 10.11 wt.%. The theoretical BeO content, calculated from stoichiometry, is ~9.0 wt.%. This discrepancy, ~10 rel.%, is still within the analytical error of LA-ICP-MS for Be (~10 rel.%). The average content of B2O3 is 0.10 wt.%. Laser ablation ICP-MS also revealed very low U and Th contents in the gadolinite (~80 and ~5 ppm, respectively). Such a low radioactive element content does not affect the crystallinity of the mineral. A small amount of OH (0.34 pfu) resulted from the calculation of an empirical formula. The empirical formula obtained from the mean of three analyses of the crystal used for structural determination is:
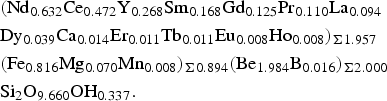 $$\eqalign{& ({\rm N}{\rm d}_{0.632}{\rm C}{\rm e}_{0.472}{\rm Y}_{0.268}{\rm S}{\rm m}_{0.168}{\rm G}{\rm d}_{0.125}{\rm Pr}_{0.110}{\rm L}{\rm a}_{0.094} \cr & {\rm D}{\rm y}_{0.039}{\rm C}{\rm a}_{0.014}{\rm E}{\rm r}_{0.011}{\rm T}{\rm b}_{0.011}{\rm E}{\rm u}_{0.008}{\rm H}{\rm o}_{0.008})_{\Sigma 1.957} \cr & ({\rm F}{\rm e}_{0.816}{\rm M}{\rm g}_{0.070}{\rm M}{\rm n}_{0.008})_{\Sigma 0.894}({\rm B}{\rm e}_{1.984}{\rm B}_{0.016})_{\Sigma 2.000} \cr & {\rm S}{\rm i}_2{\rm O}_{9.660}{\rm O}{\rm H}_{0.337}.} $$
$$\eqalign{& ({\rm N}{\rm d}_{0.632}{\rm C}{\rm e}_{0.472}{\rm Y}_{0.268}{\rm S}{\rm m}_{0.168}{\rm G}{\rm d}_{0.125}{\rm Pr}_{0.110}{\rm L}{\rm a}_{0.094} \cr & {\rm D}{\rm y}_{0.039}{\rm C}{\rm a}_{0.014}{\rm E}{\rm r}_{0.011}{\rm T}{\rm b}_{0.011}{\rm E}{\rm u}_{0.008}{\rm H}{\rm o}_{0.008})_{\Sigma 1.957} \cr & ({\rm F}{\rm e}_{0.816}{\rm M}{\rm g}_{0.070}{\rm M}{\rm n}_{0.008})_{\Sigma 0.894}({\rm B}{\rm e}_{1.984}{\rm B}_{0.016})_{\Sigma 2.000} \cr & {\rm S}{\rm i}_2{\rm O}_{9.660}{\rm O}{\rm H}_{0.337}.} $$The ideal formula is Nd2FeBe2Si2O10, which requires 58.16 wt.% Nd2O3, 12.42 wt.% FeO, 8.65 wt.% BeO and 20.77 wt.% SiO2.
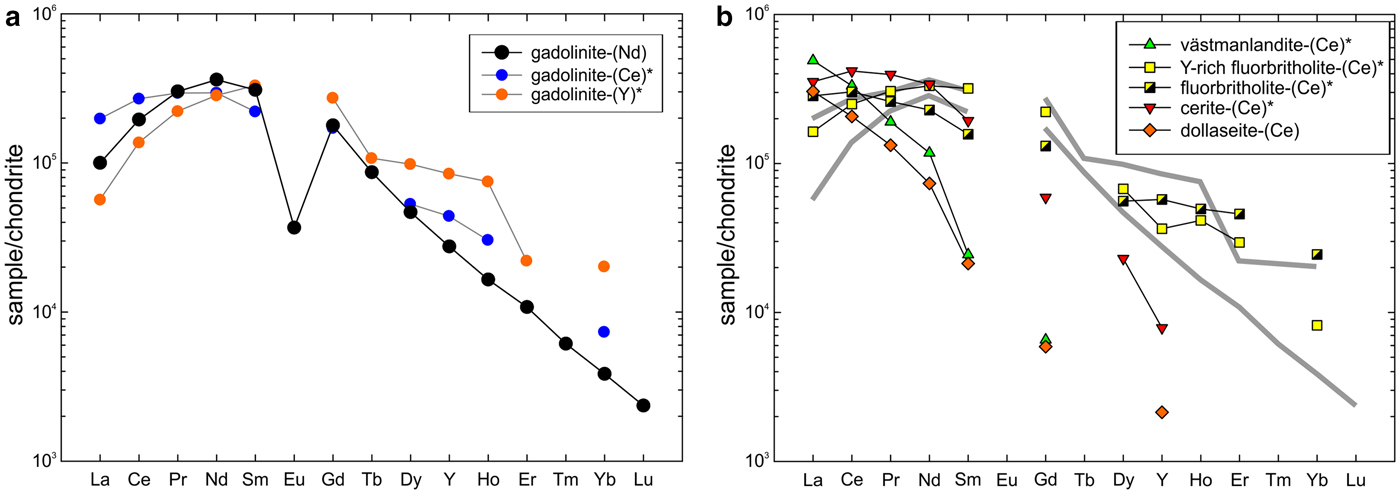
Fig. 2. (a) Chondrite-normalized REE pattern of gadolinite-(Nd) obtained as a combination of EPMA and LA-ICP-MS data. Patterns of gadolinite-(Ce) and gadolinite-(Y) from Holtstam and Andersson (Reference Holtstam and Andersson2007) are plotted for comparison. (b) Patterns of other REE-silicates from Malmkärra are plotted for visualization of distribution of REE among individual structural types. Grey, thick, lines indicate the compositional field of gadolinite-subgroup minerals. Chondrite values of McDonough and Sun (Reference McDonough and Sun1995) were used for normalization. Data for minerals indicated by an asterisk are taken from Holtstam and Andersson (Reference Holtstam and Andersson2007).
Table 3. Chemical composition of the gadolinite-(Nd) based on the EMP and LA-ICP-MS data.
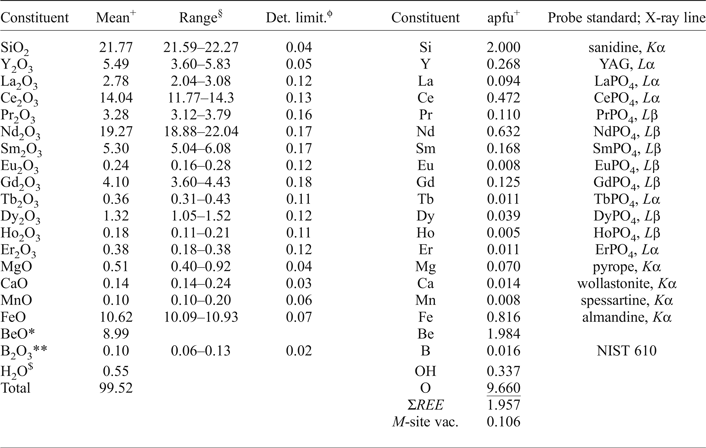
+Mean of three analyses of the crystal used for structural determination; apfu = atoms per formula unit; *determined from stoichiometry; §range of all (11) analyses; **measured by LA-ICP-MS; ϕdetection limit in oxides; $calculated from charge balance.
Table 4. LA-ICP-MS data for gadolinite-(Nd) from Malmkärra (in ppm).
A part of the largest grain (150 µm × 100 µm) of gadolinite-(Nd), carefully measured by EPMA, was extracted subsequently from the thin section for the single-crystal X-ray diffraction study. The other parts of this grain were used for determination of the optical properties.
Raman spectroscopy
Raman analysis of gadolinite-(Nd), performed on uncoated polished sections, was undertaken by means of a Horiba LabRAM HR Evolution spectrometer. This dispersive, edge-filter-based system was equipped with an Olympus BX 41 optical microscope, a diffraction grating with 600 grooves per millimetre, and a Peltier-cooled, Si-based charge-coupled device (CCD) detector. Raman spectra of REE-bearing minerals are commonly obscured by laser-induced emissions of the REE (e.g. Nasdala et al., Reference Nasdala, Beyssac, Schopf, Bleisteiner and Zoubrir2012; Lenz et al., Reference Lenz, Nasdala, Talla, Hauzenberger, Seitz and Kolitsch2015). After careful tests with different lasers (473, 523 and 633 nm), the 633 nm He-Ne laser (10 mW at the sample surface) was selected for spectral acquisition to minimize analytical artefacts. With the Olympus 100× objective (numerical aperture 0.9) and the system being operated in the confocal mode, the lateral resolution was ~1 μm. Wavenumber calibration was done using the Rayleigh line and Ne-lamp emissions. The wavenumber accuracy was >0.5 cm–1 and the spectral resolution was ~2 cm–1. Band fitting was done after appropriate background correction, assuming combined Lorentzian-Gaussian band shapes using PeakFit (Jandel Scientific Software).
The Raman spectrum of gadolinite-(Nd) is shown in Fig. 3. Following the spectroscopic study of isostructural datolite and herderite (Frost et al., Reference Frost, Xi, Scholz, Lima, Horta and Lopez2013, Reference Frost, Scholz, López, Xi, de Siqueira Queiroz, Belotti and Cândido Filho2014; Goryainov et al., Reference Goryainov, Krylov, Vtyurin and Pan2015) the most intense Raman bands at 897 and 970 cm–1 are assigned to stretching vibrations of Be–O and Si–O in tetrahedral coordination. The bands in the region 200–750 cm–1 are assigned to banding modes of Si–O and Be–O, stretching vibrations of REE–O and Fe–O and to lattice vibrations. The weak band at 3525 cm–1 is attributed to stretching vibrations of OH units in the gadolinite structure. The local maximum at 3370 cm–1 is caused by luminescence as proven by excitation using a 532 nm laser.

Fig. 3. Raman spectrum of gadolinite-(Nd) excited by a 633 nm laser.
X-ray crystallography and structure determination
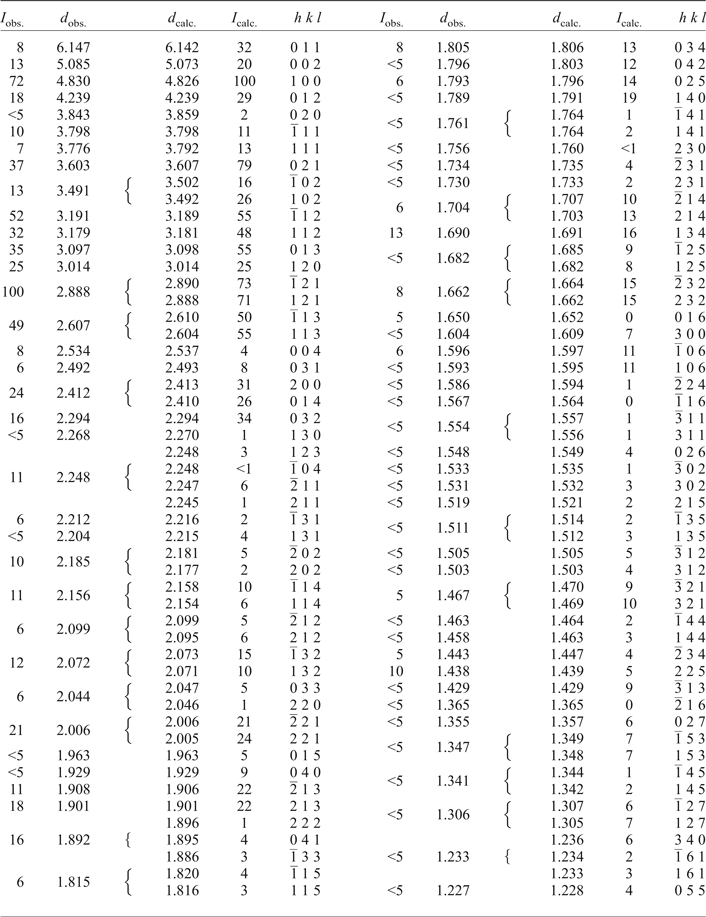
Powder X-ray diffraction data for gadolinite-(Nd), carefully extracted from the polished section by a diamond microgrinder and powdered under isopropyl alcohol in an agate mortar, were obtained using an Empyrean (PANalytical) powder diffractometer with the solid-state PIXcel3D detector operating in Bragg-Brentano geometry. Data were collected over the range of 5–80°2θ with a step size of 0.013° and counting time of 2 s per step. Positions and intensities of diffraction maxima were obtained from a profile fitting performed with High-Score3 software (PANalytical). Unit-cell parameters were refined using least-squares based on the positions of 40 reflections utilizing Celref software (Laugier and Bochu, Reference Laugier and Bochu2004). The experimental powder pattern was indexed in accordance with the calculated values of intensities obtained from the single-crystal structure refinement. Powder X-ray diffraction data are given in Table 5. Unit-cell parameters refined from the powder data are a = 4.826(1), b = 7.717(2), c = 10.147(2) Å, β = 90.16° and V = 377.9(2) Å3.
Table 5. Powder XRD data of gadolinite-(Nd) from Malmkärra.
A platy crystal fragment (0.07 mm × 0.06 mm × 0.01 mm) was examined on an Oxford Diffraction Gemini single-crystal diffractometer with Atlas S2 CCD detector, using monochromatized MoKα radiation (λ = 0.71073 Å) from a conventional sealed X-ray tube and collimated with fibre optics and Mo-Enhance collimator. The unit cell was refined from 1323 reflections using least-squares with the Crysalis software (2014, Agilent Technologies, Oxford, UK); ω rotational scans (frame width 1°, counting time 170 s per frame) were adopted to collect a set of three-dimensional intensity data. From a total of 2955 reflections, 875 were independent and 607 classified as observed, with [I obs > 3σ(I)]. Corrections for background, Lorentz effects and polarization were applied; Gaussian correction for absorption (shape + empirical scaling) was done in JANA2006 (Petříček et al., Reference Petříček, Dušek and Palatinus2014) giving a data set with R int of ~10%. Reduction of the data was performed using the Crysalis package. The crystal structure of gadolinite-(Nd) was refined from single-crystal data using the structure model for gadolinite-(Y) published in Demartin et al. (Reference Demartin, Pilati, Diella, Gentile and Gramaccioli1993) by full-matrix least-squares using JANA2006 (Petříček et al., Reference Petříček, Dušek and Palatinus2014) based on F 2. All non-oxygen atoms were refined with anisotropic displacement parameters. Refinement for 57 parameters and 18 constraints converged to R = 0.0371, wR = 0.0780 for 607 observed reflections (Goof = 1.14). Details of data collection, crystallographic data and refinement details are given in Table 6. Atom coordinates and displacement parameters are listed in Table 7; selected interatomic distances are provided in Table 8.
Table 6. Summary of data collection conditions and refinement parameters for gadolinite-(Nd).

Table 7. Atom positions, displacement parameters (equivalent and anisotropic, in Å2) and bond valences (in valence-units) for gadolinite-(Nd).

#refined occupancies for Si1/Be1 are 0.982(17)/0.018(17) and for Be2/Si2 0.947(16)/0.053(16).
*refined with isotropic displacement parameter.
Table 8. Selected interatomic distances (in Å) in the structure of gadolinite-(Nd).
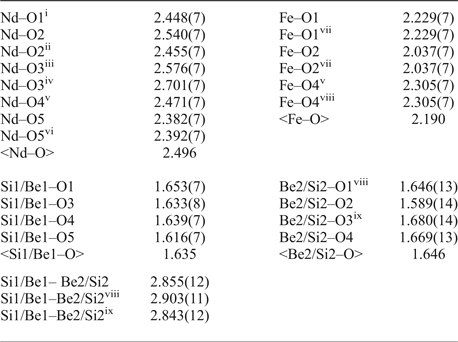
Symmetry codes: (i) x, –y + 1/2, z + 1/2; (ii) –x + 2, y–1/2, –z + 3/2; (iii) –x + 1, –y, –z + 1; (iv) x + 1, –y + 1/2, z + 1/2; (v) x + 1, y, z; (vi) –x + 2, –y, –z + 1; (vii) –x + 2, –y + 1, –z + 1; (viii) –x + 1, –y + 1, –z + 1; (ix) x, –y + 1/2, z–1/2; (x) –x + 1, y + 1/2, –z + 1/2.
The structure refinement of gadolinite-(Nd) provides no significant departure from the published structure results of the isostructural gadolinite-(Y) (Miyawaki et al., Reference Miyawaki, Nakai and Nagashima1984; Demartin et al., Reference Demartin, Pilati, Diella, Gentile and Gramaccioli1993; Cámara et al., Reference Cámara, Oberti, Ottolini, Della Ventura and Bellatreccia2008). Minerals of the gadolinite group possess a layer structure composed of two distinct sheets, which are alternating in the c direction (Bačík et al., Reference Bačík, Fridrichová, Uher, Pršek and Ondrejka2014). One sheet is represented by TO4 and QO4 tetrahedra and it alternates with a second sheet of AO8 polyhedra and MO6 octahedra. In the structure of gadolinite-(Nd), the T-site is occupied dominantly by Si4+, the Q-site by Be, the A-site by Nd3+ (and other REE) and the M-site by Fe2+. During the refinement we treated A-sites as only occupied by Nd. We did not model substitutions of a large number of REE that have very similar scattering curves for X-rays as we are convinced that such a fit would not make much sense. The refined site occupancies for Nd and Be turned out they be slightly lower than unity (due to the effects of mixing with lighter elements at particular sites). The Mg substitution for Fe, as documented from the wavelength-dispersive spectroscopy analyses, was not successfully confirmed by the structure refinement. The structural formula for gadolinite-(Nd) is therefore Nd1.942FeBe1.931Si2.069O10, Z = 2. This formula is not electro-neutral due to the fact that the refined occupancies are only a proxy to the real situation, the refinement of all (excluding REE) substituting components is beyond the quality of current dataset. However, the refinement of the data suggested that a small amount of Si can enter the Q-sites and vice-versa.
Concluding remarks
Rare-earth element ores from the Bastnäs-type deposits are in general characterized by LREE enriched and HREE depleted patterns (e.g. Holtstam et al., Reference Holtstam, Andersson, Broman and Mansfeld2014). Taking into consideration the low activity of P and elevated activity of CO2, the primary REE minerals are represented as silicates and carbonates. Among the REE phases of the Bastnäs-type mineralization at the Malmkärra mine, the gadolinite-group minerals together with fluorbritholite represent the phases which accommodate the highest amount of middle-rare-earth elements (MREE) and HREE into their structure. On the other hand, epidote-supergroup minerals, including västmanlandite, are strongly depleted in MREE and HREE (Fig. 2).
Notably, the new mineral may represent a locally important sink for the critical metal neodymium in deposits of the Bastnäs-type. Geijer (Reference Geijer1936) reported törnebohmite as occurring locally in “not insignificant amounts” at Malmkärra, while Holtstam and Andersson (Reference Holtstam and Andersson2007) make no mention of having observed this mineral. Considering their, in part similar, optical properties, it seems possible that Geijer's törnebohmite could represent (a misidentified) gadolinite. Additionally, new observations also suggest that it may be more abundant than hitherto known in other Bastnäs-type mineralizations, specifically in the Johanna and Nya Bastnäs mines.
Acknowledgements
The authors are grateful to Peter Bačík, Daniel Atencio, an anonymous reviewer and the Structures Editor Peter Leverett for constructive criticism that significantly improved the manuscript, as well as to Editor John Bowles for careful handling of the manuscript. Voting members of the IMA-CNMNC are acknowledged for the constructive remarks to the proposal. This study was supported by the research projects GAČR P210/14/1347S and MUNI/A/1005/2013 for RŠ and RČ and MN. Single-crystal X-ray diffraction experiments were done using instruments of the ASTRA lab established within the Operation program Prague Competitiveness – project CZ.2.16/3.1.00/24510. EJ acknowledges support from the Swedish Research Council (VR) and the Geological Survey of Sweden (SGU) to projects on rare and critical metals in central Sweden at Uppsala University. MVG acknowledges CEITEC 2020 (LQ1601) project with financial contribution made by the Ministry of Education, Youth and Sports of the Czech Republic within special support paid from the National Program for Sustainability II Funds.















