Article contents
Kazanskyite, Ba & TiNbNa3Ti(Si2O7)2O2(OH)2(H2O)4, a Group-III Ti-disilicate mineral from the Khibiny alkaline massif, Kola Peninsula, Russia: description and crystal structure
Published online by Cambridge University Press: 05 July 2018
Abstract
Kazanskyite, Ba & TiNbNa3Ti(Si2O7)2O2(OH)2(H2O)4, is a Group-III TS-block mineral from the Kirovskii mine, Mount Kukisvumchorr, Khibiny alkaline massif, Kola Peninsula, Russia. The mineral occurs as flexible and commonly bent flakes 2–15 μm thick and up to 330 μm across. It is colourless to pale tan, with a white streak and a vitreous lustre. The mineral formed in a pegmatite as a result of hydrothermal activity. Associated minerals are natrolite, barytolamprophyllite, nechelyustovite, hydroxylapatite, belovite-(La), belovite-(Ce), gaidonnayite, nenadkevichite, epididymite, apophyllite-(KF) and sphalerite. Kazanskyite has perfect cleavage on {001}, splintery fracture and a Mohs hardness of 3. Its calculated density is 2.930 g cm–3. Kazanskyite is biaxial positive with α 1.695, β 1.703, γ 1.733 (λ590 nm), 2Vmeas = 64.8(7)°, 2Vcalc = 55.4°, with no discernible dispersion. It is not pleochroic. Kazanskyite is triclinic, space group P , a 5.4260(9), b 7.135(1), c 25.514(4) Å, α 90.172(4), β 90.916(4), γ 89.964(3)°, V 977.61(3) Å3. The strongest lines in the X-ray powder-diffraction pattern [d(Å)(I)(hkl)] are: 2.813(100)(12
, a 5.4260(9), b 7.135(1), c 25.514(4) Å, α 90.172(4), β 90.916(4), γ 89.964(3)°, V 977.61(3) Å3. The strongest lines in the X-ray powder-diffraction pattern [d(Å)(I)(hkl)] are: 2.813(100)(12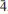 ,1
,1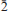
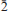 ), 2.149(82)(22
), 2.149(82)(22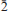 ,2
,2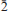 0,207,220,2
0,207,220,2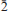 2), 3.938(70)(1
2), 3.938(70)(1 3,112), 4.288(44)(11
3,112), 4.288(44)(11 ,1
,1 0,110,1
0,110,1 1), 2.128(44)(22
1), 2.128(44)(22 ,2
,2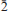
 ,1
,1 4,221,1
4,221,1 4,221,2
4,221,2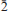 3), 3.127(39)(1
3), 3.127(39)(1 6,115), 3.690(36)(1
6,115), 3.690(36)(1 4), 2.895(33)(1
4), 2.895(33)(1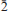 3,121) and 2.955(32)(1
3,121) and 2.955(32)(1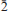 0,120,1
0,120,1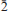 2). Chemical analysis by electron microprobe gave Nb2O59.70, TiO219.41, SiO228.21, Al2O30.13, FeO 0.28, MnO 4.65, BaO 12.50, SrO 3.41, CaO 0.89, K2O 1.12, Na2O 9.15, H2O 9.87, F 1.29, O = F –0.54, sum 100.07 wt.%; H2O was determined from structure refinement. The empirical formula is (Na2.55Mn0.31Ca0.11Fe2+0.03)Σ3(Ba0.70Sr0.28K0.21Ca0.03)Σ1.22(Ti2.09Nb0.63Mn0.26Al0.02)Σ3Si4.05O21.42H9.45F0.59, calculated on 22 (O + F) a.p.f.u., Z = 2. The structural formula of the form AP2MH2MO4(Si2O7)2XO4XPMXPA(H2O)n is (Ba0.56Sr0.22K0.15Ca0.03☐0.04)Σ1(☐0.74Ba0.14Sr0.06K0.06)Σ1(Ti0.98Al0.0 2)Σ1(Nb0.63Ti0.37)Σ1(Na2.55Mn0.31Ca0.11Fe2+0.03)Σ3(Ti0.74Mn0.26)Σ1(Si2O7)2O2(OH1.41F0.59)Σ2(H2O)(☐0.74H2O0.26)Σ1(H2O)2.74. Simplified and ideal formulae are as follows: Ba(☐,Ba)Ti(Nb,Ti)(Na,Mn)3(Ti,Mn)(Si2O7)2O2(OH,F)2(H2O)4 and Ba☐TiNbNa3Ti(Si2O7)2O2(OH)2(H2O)4. The Raman spectrum of the mineral contains the following bands: 3462 cm–1 (broad) and 3545 and 3628 cm–1 (sharp). The crystal structure was solved by direct methods and refined to an R1 index of 8.09%. The crystal structure of kazanskyite is a combination of a TS (titanium silicate) block and an I (intermediate) block. The TS block consists of HOH sheets (H is heteropolyhedral and O is octahedral). The TS block exhibits linkage and stereochemistry typical for Group-III (Ti = 3 a.p.f.u.) Ti-disilicate minerals. The TS block has two different H sheets where (Si2O7) groups link to [5]-coordinated Ti and [6]-coordinated Nb polyhedra, respectively. There are two peripheral sites, AP(1,2), occupied mainly by Ba (less Sr and K) at 96% and 26%. There are two I blocks: the I1 block is a layer of Ba atoms; the I2 block consists of H2O groups and AP(2) atoms. The TS and I blocks are topologically identical to those in the nechelyustovite structure. The mineral is named in honour of Professor Vadim Ivanovich Kazansky, a prominent Russian ore geologist and an expert in Precambrian metallogeny.
2). Chemical analysis by electron microprobe gave Nb2O59.70, TiO219.41, SiO228.21, Al2O30.13, FeO 0.28, MnO 4.65, BaO 12.50, SrO 3.41, CaO 0.89, K2O 1.12, Na2O 9.15, H2O 9.87, F 1.29, O = F –0.54, sum 100.07 wt.%; H2O was determined from structure refinement. The empirical formula is (Na2.55Mn0.31Ca0.11Fe2+0.03)Σ3(Ba0.70Sr0.28K0.21Ca0.03)Σ1.22(Ti2.09Nb0.63Mn0.26Al0.02)Σ3Si4.05O21.42H9.45F0.59, calculated on 22 (O + F) a.p.f.u., Z = 2. The structural formula of the form AP2MH2MO4(Si2O7)2XO4XPMXPA(H2O)n is (Ba0.56Sr0.22K0.15Ca0.03☐0.04)Σ1(☐0.74Ba0.14Sr0.06K0.06)Σ1(Ti0.98Al0.0 2)Σ1(Nb0.63Ti0.37)Σ1(Na2.55Mn0.31Ca0.11Fe2+0.03)Σ3(Ti0.74Mn0.26)Σ1(Si2O7)2O2(OH1.41F0.59)Σ2(H2O)(☐0.74H2O0.26)Σ1(H2O)2.74. Simplified and ideal formulae are as follows: Ba(☐,Ba)Ti(Nb,Ti)(Na,Mn)3(Ti,Mn)(Si2O7)2O2(OH,F)2(H2O)4 and Ba☐TiNbNa3Ti(Si2O7)2O2(OH)2(H2O)4. The Raman spectrum of the mineral contains the following bands: 3462 cm–1 (broad) and 3545 and 3628 cm–1 (sharp). The crystal structure was solved by direct methods and refined to an R1 index of 8.09%. The crystal structure of kazanskyite is a combination of a TS (titanium silicate) block and an I (intermediate) block. The TS block consists of HOH sheets (H is heteropolyhedral and O is octahedral). The TS block exhibits linkage and stereochemistry typical for Group-III (Ti = 3 a.p.f.u.) Ti-disilicate minerals. The TS block has two different H sheets where (Si2O7) groups link to [5]-coordinated Ti and [6]-coordinated Nb polyhedra, respectively. There are two peripheral sites, AP(1,2), occupied mainly by Ba (less Sr and K) at 96% and 26%. There are two I blocks: the I1 block is a layer of Ba atoms; the I2 block consists of H2O groups and AP(2) atoms. The TS and I blocks are topologically identical to those in the nechelyustovite structure. The mineral is named in honour of Professor Vadim Ivanovich Kazansky, a prominent Russian ore geologist and an expert in Precambrian metallogeny.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2016
References
- 12
- Cited by


