Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Valsami-Jones, E.
and
Manning, D. A. C.
2003.
Environmental Mineralogy: introduction to a thematic set of papers arising out of sessions held at IMA 2002, Edinburgh, UK.
Mineralogical Magazine,
Vol. 67,
Issue. 6,
p.
1123.
Freij, S.J.
Putnis, A.
and
Astilleros, J.M.
2004.
Nanoscale observations of the effect of cobalt on calcite growth and dissolution.
Journal of Crystal Growth,
Vol. 267,
Issue. 1-2,
p.
288.
Freij, Sawsan J.
Godelitsas, Athanasios
and
Putnis, Andrew
2005.
Crystal growth and dissolution processes at the calcite–water interface in the presence of zinc ions.
Journal of Crystal Growth,
Vol. 273,
Issue. 3-4,
p.
535.
Shaver, Stephen A.
Hower, James C.
Eble, Cortland F.
McLamb, Elizabeth D.
and
Kuers, Karen
2006.
Trace element geochemistry and surface water chemistry of the Bon Air coal, Franklin County, Cumberland Plateau, southeast Tennessee.
International Journal of Coal Geology,
Vol. 67,
Issue. 1-2,
p.
47.
Vavouraki, Aikaterini I.
Putnis, Christine V.
Putnis, Andrew
and
Koutsoukos, Petros G.
2010.
Crystal Growth and Dissolution of Calcite in the Presence of Fluoride Ions: An Atomic Force Microscopy Study.
Crystal Growth & Design,
Vol. 10,
Issue. 1,
p.
60.
Astilleros, J.M.
Godelitsas, A.
Rodríguez-Blanco, J.D.
Fernández-Díaz, L.
Prieto, M.
Lagoyannis, A.
and
Harissopulos, S.
2010.
Interaction of gypsum with lead in aqueous solutions.
Applied Geochemistry,
Vol. 25,
Issue. 7,
p.
1008.
Godelitsas, Athanasios
and
Astilleros, José M.
2011.
Ion Partitioning in Ambient-Temperature Aqueous Systems.
p.
289.
-Agudo, E. Ruiz
and
Putnis, C. V.
2012.
Direct observations of mineral fluid reactions using atomic force microscopy: the specific example of calcite.
Mineralogical Magazine,
Vol. 76,
Issue. 1,
p.
227.
Rouff, Ashaki A.
2012.
Sorption of Chromium with Struvite During Phosphorus Recovery.
Environmental Science & Technology,
Vol. 46,
Issue. 22,
p.
12493.
Peña-Rodríguez, Susana
Bermúdez-Couso, Alipio
Nóvoa-Muñoz, Juan Carlos
Arias-Estévez, Manuel
Fernández-Sanjurjo, María J.
Álvarez-Rodríguez, Esperanza
and
Núñez-Delgado, Avelino
2013.
Mercury removal using ground and calcined mussel shell.
Journal of Environmental Sciences,
Vol. 25,
Issue. 12,
p.
2476.
Wang, Peng
Hudak, Michael R.
Lerner, Allan
Grubbs, Robert K.
Wang, Shanmin
Zhang, Zhan
Karapetrova, Evguenia
Hickmott, Donald
and
Majewski, Jaroslaw
2014.
X-ray scattering of calcite thin films deposited by atomic layer deposition: Studies in air and in calcite saturated water solution.
Thin Solid Films,
Vol. 565,
Issue. ,
p.
277.
Bracco, Jacquelyn N.
Lee, Sang Soo
Stubbs, Joanne E.
Eng, Peter J.
Jindra, Sarah
Warren, D. Morgan
Kommu, Anitha
Fenter, Paul
Kubicki, James D.
and
Stack, Andrew G.
2019.
Simultaneous Adsorption and Incorporation of Sr2+ at the Barite (001)–Water Interface.
The Journal of Physical Chemistry C,
Vol. 123,
Issue. 2,
p.
1194.
Tang, Hongmei
Xian, Haiyang
He, Hongping
Wei, Jingming
Liu, Hongmei
Zhu, Jianxi
and
Zhu, Runliang
2019.
Kinetics and mechanisms of the interaction between the calcite (10.4) surface and Cu2+-bearing solutions.
Science of The Total Environment,
Vol. 668,
Issue. ,
p.
602.
Chukanov, Nikita V.
and
Vigasina, Marina F.
2020.
Vibrational (Infrared and Raman) Spectra of Minerals and Related Compounds.
p.
19.
Di Lorenzo, Fulvio
Steiner, Kay
and
Churakov, Sergey V.
2021.
The Effect of pH, Ionic Strength and the Presence of PbII on the Formation of Calcium Carbonate from Homogenous Alkaline Solutions at Room Temperature.
Minerals,
Vol. 11,
Issue. 7,
p.
783.
 4} cleavage surface of calcite with Hg(CH3COO)2 aqueous solutions with concentration of 5 mM Hg(II) (pH ≈3.5), was investigated using microscopic and spectroscopic techniques. In situ atomic force microscopy experiments showed that surface microtopography changes significantly as a result of the interaction, and that the initial rhombic etch pits induced by H2O dissolution are rapidly transformed to deeper etch pits exhibiting an unusual triangular shape. The growth of these etch pits is strongly anisotropic, moving faster along the [2
4} cleavage surface of calcite with Hg(CH3COO)2 aqueous solutions with concentration of 5 mM Hg(II) (pH ≈3.5), was investigated using microscopic and spectroscopic techniques. In situ atomic force microscopy experiments showed that surface microtopography changes significantly as a result of the interaction, and that the initial rhombic etch pits induced by H2O dissolution are rapidly transformed to deeper etch pits exhibiting an unusual triangular shape. The growth of these etch pits is strongly anisotropic, moving faster along the [2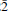 1] direction than along the [010] direction (with step-retreat velocities of ∼12 nm s –1 and ∼4 nm s–1, respectively). The modified etch pits are due to Hg(II) sorption in the surface, rather than due to the effect of the acetate anion. The sorption (adsorption and probably absorption also) of Hg(II), in the first minutes of the interaction, is shown by X-ray photoelectron spectroscopy. After ∼2 h, the triangular etch pits are interconnected to form larger hexagonal etch pits, while Hg(II)-bearing phases (confirmed later by SEM-EDS) grow onto the surface through a heterogeneous nucleation process. The crystal growth of orthorhombic (montroydite-type) hydrated Hg(II) oxide (HgO·nH2O) on the surface of calcite was confirmed by XRD patterns and FT-IR spectra from samples exposed for longer times to Hg(CH3COO)2 solution.
1] direction than along the [010] direction (with step-retreat velocities of ∼12 nm s –1 and ∼4 nm s–1, respectively). The modified etch pits are due to Hg(II) sorption in the surface, rather than due to the effect of the acetate anion. The sorption (adsorption and probably absorption also) of Hg(II), in the first minutes of the interaction, is shown by X-ray photoelectron spectroscopy. After ∼2 h, the triangular etch pits are interconnected to form larger hexagonal etch pits, while Hg(II)-bearing phases (confirmed later by SEM-EDS) grow onto the surface through a heterogeneous nucleation process. The crystal growth of orthorhombic (montroydite-type) hydrated Hg(II) oxide (HgO·nH2O) on the surface of calcite was confirmed by XRD patterns and FT-IR spectra from samples exposed for longer times to Hg(CH3COO)2 solution.



