Article contents
Thermochemistry of Rare Earth Perovskites
Published online by Cambridge University Press: 30 June 2016
Abstract
The rare earth (RE) mineral loparite with the chemical composition (RE, Na, Sr, Ca)(Ti, Nb, Ta, Fe+3)O3 is the principal ore of the light rare earth elements (LREE) as well as niobium and tantalum. The enthalpies of formation of RE0.67-xNa3xTiO3 (RE = La, Ce) and Ca1-2xNaxLaxTiO3 from oxides and elements of lanthanum and cerium perovskites and their solid solutions have been obtained using high temperature oxide melt solution calorimetry. RE0.67-xNa3xTiO3 (RE = La, Ce) perovskites become more stable relative to oxide components as sodium content increases. Na0.5Ce0.5TiO3 and Na0.5La0.5TiO3 can be considered stable endmembers in natural loparite minerals. For perovskite solid solutions Ca1-2xNaxLaxTiO3, the enthalpies of formation from the constituent oxides
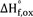 $\Delta {\rm{H}}_{{\rm{f}},\,{\rm{ox}}}^^\circ$
become more exothermic with increasing Na+La content, suggesting a stabilizing effect of the substitution 2Ca2+ → Na+ + La3+ on the perovskite structure. The trend of increasing thermodynamic stability with decreasing structural distortion is similar to that seen in many other ABO3 perovskites.
$\Delta {\rm{H}}_{{\rm{f}},\,{\rm{ox}}}^^\circ$
become more exothermic with increasing Na+La content, suggesting a stabilizing effect of the substitution 2Ca2+ → Na+ + La3+ on the perovskite structure. The trend of increasing thermodynamic stability with decreasing structural distortion is similar to that seen in many other ABO3 perovskites.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2016
References
- 4
- Cited by




