Only 2.5% of all the water on Earth is freshwater, and less than 1% of this freshwater is usable by humans. This scarcity, accompanied by over-usage, uneven water distribution, and uncertain weather patterns, calls for cost-effective technologies to acquire freshwater. Solar desalination, a technology that uses solar energy to boil saline water and condense the resulting steam to fresh water, is a sustainable solution to the freshwater crisis. Unfortunately, inefficient solar-energy harvesting leads to insufficient energy input to evaporate large amounts of water, and sluggish desalination rates (typically ≤1.6 kg m–2 h–1).
A research team led by Guihua Yu of The University of Texas at Austin has broken the performance ceiling of solar desalination. The researchers developed hydrogels consisting of poly(vinyl alcohol) (PVA) and chitosan as hydrophilic polymeric networks, and polypyrrole as a solar light absorber. A desalination rate of ∼3.6 kg m–2 h–1 under 1-sun illumination (100 mW cm–2) was achieved, which is more than double the typical value achieved so far. These results were published in a recent issue of Science Advances (doi:10.1126/sciadv.aaw5484).
The hydrophilic nature of the PVA/chitosan hydrogel reduced the energy required to evaporate water. “Hydroxyl groups (–OH) in PVA, along with –OH and amino groups in chitosan can capture water molecules with hydrogen bonds and create intermediate water for fast evaporation,” says Xingyi Zhou, the lead author. “Intermediate water” refers to water molecules other than those bound tightly to the polymers and free molecules in bulk water. The effortless heat transfer from the polymer backbones to the intermediate water molecules reduced the enthalpy of water vaporization.
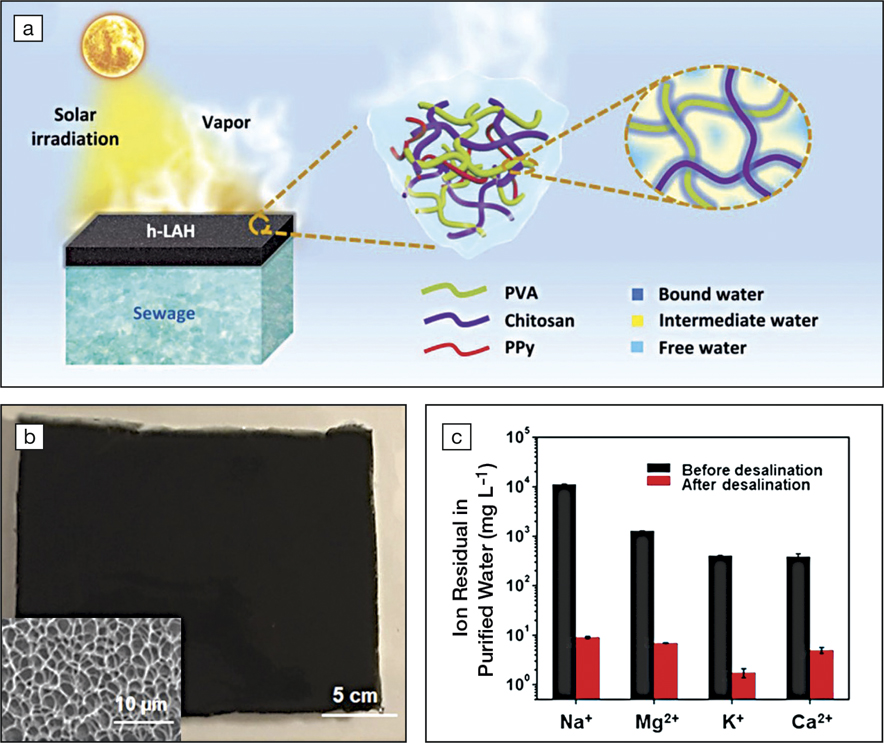
a) Schematic illustration of solar desalination using poly(vinyl alcohol) (PVA)/chitosan hydrogels. h-LAH, hydratable light-absorbing hydrogel; PPy, polypyrrole. (b) Photograph of a piece of PVA/chitosan hydrogel. Inset: Scanning electron microscope image showing the microstructure of the hydrogel. (c) Ion concentrations of seawater (Gulf of Mexico) before and after solar desalination (note logarithmic scale of y-axis). Credit: Science Advances.
The PVA/chitosan hydrogel exhibited outstanding water desalination performance. By desalinating seawater from the Gulf of Mexico, the hydrogel decreased the concentrations of Na+, Mg2+, K+, and Ca2+ by approximately 2–3 orders of magnitude, reaching values lower than those obtained using membrane-based desalination techniques (e.g., reverse osmosis). Additionally, the hydrogel exhibited a consistently high desalination capacity of ∼3.6 kg m–2 h–1 under continuous sun irradiation for more than four days.
According to Jia Zhu of Nanjing University, China, whose group is working on solar heat management and conversion, this work may open new opportunities to design high-performance solar desalination materials. He says, “Converting bulk water to intermediate water is intriguing. It could also inspire future works following the path set by this work—to gain knowledge about the water molecule behaviors at the nanoscale.” Zhu was not involved in this study.
Although the scalability and desalination capacity of solar desalination is not competitive when compared to reverse osmosis and multistage distillation, the researchers are optimistic because solar desalination demands only sunlight as the energy input. “Due to the easy access to solar energy, we envision that our material can enable small-scale and portable solar water purification devices. The fast water production rate under natural sunlight [will be useful] for emergency need[s],” says Fei Zhao, the co-first author of the work. “Higher yield of low-salinity water is achievable by further optimizing the hydrogel materials through strategies such as architecting polymer networks and modifying hydrogel surfaces,” he added.


