No CrossRef data available.
Article contents
Development of ionic-imprinted polyesters of diallyl dicarboxylic acids (DAPY) for uranyl ion extraction (UO22+)
Published online by Cambridge University Press: 03 January 2019
Abstract
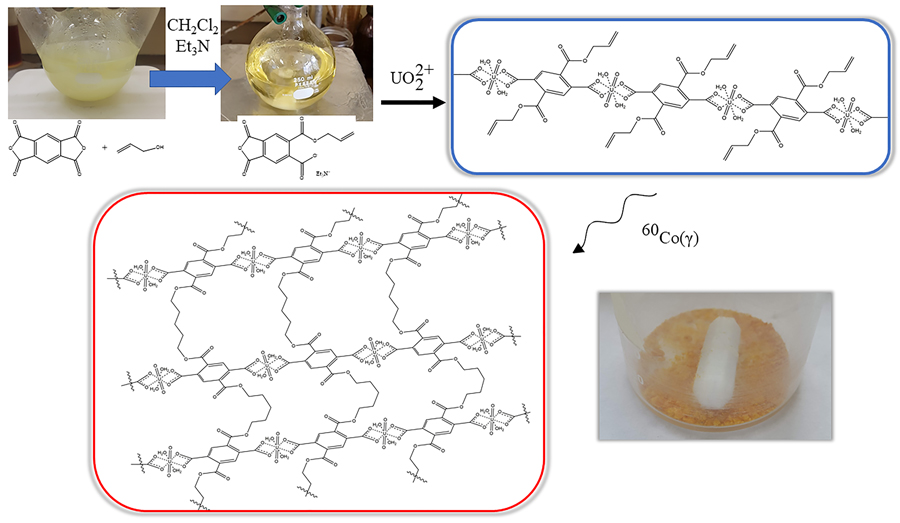
Non-conventional uranium extraction sources are not the most used mainly due to high extraction costs associated with low concentrations and chemical forms that require extra purification processes. Therefore, efforts should focus on cheaper processes and develop more effective extraction materials. In this investigation, ionic-imprinted polymers were synthesized for the selective extraction of uranyl ions in aqueous solution, using polyesters of 2,5-bis((allyloxy)carbonyl)terephthalic acid and 4,6-bis((allyloxy)carbonyl)isophthalic acid as base materials and polymerized by gamma radiation. The extraction capacity (Q) of the resins was evaluated by varying parameters such as pH, temperature, extraction time, and ionic strength.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018




