Published online by Cambridge University Press: 19 June 2019
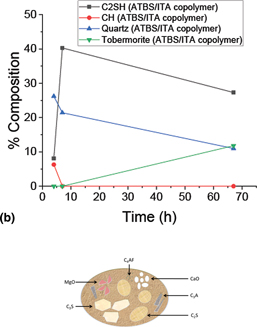
Powerful synergies between phosphonate, zinc oxide, and acrylamido-tert-butyl-sulfonate (ATBS) copolymer chemical additives render superior performance in a high-temperature retarder system for oil well grade Portland cement. The phosphonate retarder and ATBS-based retarders establish a two-tiered strength development where amorphous C-S-H converts to crystalline dicalcium silicate hydrate (C2SH) in the first (low compressive strength) tier prior to the reaction of Portlandite with quartz. The three additive retarder system can be tuned with nanosilica to eliminate the two-tiered strength development effect leading to a smooth transition from the cement in the slurry form directly to its highest compressive strength.