Article contents
Bacterial inactivation characteristics of magnesium–calcium–zinc alloys for bone implants
Published online by Cambridge University Press: 30 October 2020
Abstract
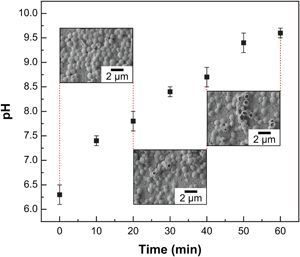
The understanding of adhesion and survival behavior of bacterial pathogens on implant surfaces are critical to control and reduce implant-associated infections. Herein, the authors investigate the interactions of Staphylococcus aureus, one of the most prevalent causes of implant infections, with Mg–4Zn–0.5Ca implants. It was found that within 60 min of exposure, 99.1% of adherent bacteria were inactivated. The combination of unique mechanical properties, biodegradation kinetics, and antimicrobial characteristics of Mg–4Zn–0.5Ca alloy makes it a promising candidate for future implant applications.
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
Footnotes
Both authors contributed equally to this work.
References
- 2
- Cited by



