Article contents
Catalysts in metal–air batteries
Published online by Cambridge University Press: 12 April 2018
Abstract
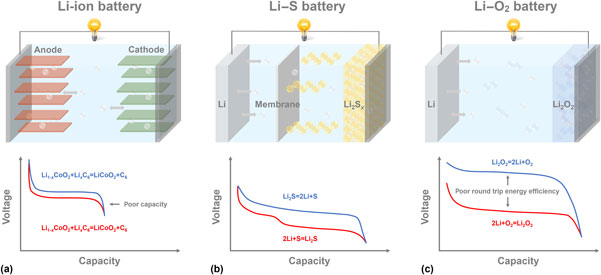
Metal–air batteries promise higher energy densities than state-of-the-art Li-ion batteries and have, therefore, received significant research attention lately. The most distinguishing feature of this technology is that it takes advantage of reversible conversion reactions of O2 or other air components (such as N2 or CO2) at the cathode. To promote these reactions, catalysts are often needed. A large number of materials have been studied for this purpose. In the present paper, we discuss the roles played by catalysts in metal–air battery systems. In particular, we choose to focus the discussions on the Li–O2 batteries as they are most intensely studied in the literature. Within this context, catalysts are often shown effective to facilitate the oxygen (O2) reduction reactions and/or O2 evolution reactions. The overall cell performance as measured by the round-trip efficiencies and charge/discharge rates can be significantly improved by the incorporation of catalysts. However, the presence of catalysts is also found to complicate the chemical reactions as they often exhibit activities toward parasitic chemical reactions such as electrolyte and electrode decompositions. The issue is especially acute in aprotic Li–O2 batteries, where organic electrolytes and reactive O2 species are mixed. In addition to heterogeneous catalysts, we also discuss the roles played by homogeneous catalysts as redox mediators, which are effective to promote redox reactions that are critical to energy storage applications.
- Type
- Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 7
- Cited by



