Article contents
Electrochemical detection of natural organic matter (humic acid) and splitting of hydrogen peroxide on a micropore 3D catalytic polysulfone–copper oxide nanocomposite surface
Published online by Cambridge University Press: 12 August 2020
Abstract
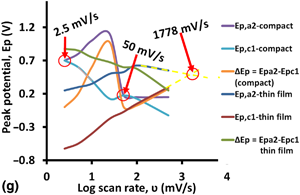
The development of new metalloplastic material from the combination of an alkaline-fused dehydrated glucose–copper (II) chloride, carbon soot, and polysulfone and the evaluation of its potential for the electrochemical detection of a pro-carcinogenic humic acid in water is reported. Excellent detection limit greater than 1 pico-part-per-million (order of 10–13 mg/L) was achieved, a value that proved to be first-of-its-kind till date. Transfer coefficients between 0.8 and 1.0 were realized. The new material showed some potential for splitting hydrogen peroxide to oxygen and thus may be explored for energy application in addition to its use as a water quality monitoring probe.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society, 2020
References
- 1
- Cited by





