No CrossRef data available.
Published online by Cambridge University Press: 02 November 2021
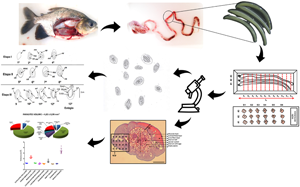
The egg is one of the fundamental parts of the life cycle of Neoechinorhynchus buttnerae, and this stage involves the acanthor larva. It is also the infection phase for the intermediate host. Under normal conditions, the larva inside the egg can survive for months in the environment; however, information regarding this phase of life of the parasite is scarce. In addition, there is no quantitative information about the structural composition of the parasite's body from a histological point of view. Such information is essential in order to support decisions aimed at controlling infestations by these parasites in fish farming. This study aimed to present a detailed description of the stages of embryonic development of N. buttnerae eggs, as well as a stereological evaluation of the body of adult females of the parasite. Three phases of development characterized the eggs: cell division (with four stages), formation of the internal nuclear mass (with four stages) and formation of the acanthor larva (with five stages). The ovary comprised 26.61% of the volume of the animal and most of it contained eggs (21.28%), ovarian balls (3.88%) and empty spaces (1.45%). These results are of great importance and will support future studies that seek to interrupt the life cycle of this parasite.