Tobacco use during adolescence continues to be a serious public health concern associated with increased mortality and personal and public costs (Substance Abuse and Mental Health Services Administration [SAMHSA], 2009). Adolescence is a critical period for the onset of cigarette use, regular cigarette use (SAMHSA, 2007), nicotine dependence (US Department of Health and Human Services, 1994), and a risk factor for the development of smoking-related illnesses (Heron et al., Reference Heron, Hickman, Macleod and Munafò2011) and other drug-related problems. Previous reviews of adult twin studies have found that additive genetic, shared and, unique environmental influences all play a role in smoking initiation (Li et al., Reference Li, Cheng, Ma and Swan2003; Sullivan & Kendler, Reference Sullivan and Kendler1999), but primarily additive and unique environmental influences influence smoking progression (i.e., developing symptoms of nicotine dependence; Kendler et al., Reference Kendler, Neale, Sullivan, Corey, Gardner and Prescott1999b; Neale et al., Reference Neale, Harvey, Maes, Sullivan and Kendler2006). When smoking progression is operationalized as number of cigarettes smoked, then a small effect of the shared environment is found for adults (Li et al., Reference Li, Cheng, Ma and Swan2003). Other works have found that there is an overlap in the genetic contribution to smoking initiation, regular tobacco use, and nicotine dependence (Maes et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004; Morley et al., Reference Morley, Lynskey, Madden, Treloar, Heath and Martin2007; Öncel et al., Reference Öncel, Dick, Maes and Alıev2014), suggesting that some of the genetic factors that influence whether individuals initiate smoking also have an impact on how many cigarettes they subsequently smoke. In addition, studies of adult twins have shown that additive genetic factors influence age at smoking initiation, but a different set of genetic factors contribute to the number of cigarettes smoked (Broms et al., Reference Broms, Silventoinen, Madden, Heath and Kaprio2006).
Work with adolescent and young adult twins has suggested that smoking initiation is primarily influenced by shared environmental factors early in adolescence (Dick et al., Reference Dick, Barman, Pitkänen, Pulkkinen, Kaprio and Rose2006; Unger et al., Reference Unger, Lessov-Schlaggar, Pang, Guo, Ning, Gallaher and Johnson2011), although some studies do suggest that additive genetic effects play a role in having ever smoked a cigarette (Huizink et al., Reference Huizink, Levälahti, Korhonen, Dick, Pulkkinen, Rose and Kaprio2010; Kendler et al., Reference Kendler, Neale, MacLean, Heath, Eaves and Kessler1993; Maes et al., Reference Maes, Woodard, Murrelle, Meyer, Silberg, Hewitt and Carbonneau1999). As adolescents reach late adolescence and young adulthood, additive genetic factors become increasingly more important (Kendler et al., Reference Kendler, Schmitt, Aggen and Prescott2008; Koopmans et al., Reference Koopmans, Doornen and Boomsma1997; Maes & Neale, Reference Maes, Neale, Swan, Baker, Chassin, Conti, Lerman and Perkins2009; Maes et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004).
Due to the conditional nature of drug use (Kendler et al., Reference Kendler, Neale, Sullivan, Corey, Gardner and Prescott1999b), properly estimating the heritability of smoking quantity requires employing a conditional model that takes into account the heritability of cigarette smoking initiation. The appropriate method to examine additive genetic and shared environmental factors to liability of drug use quantity is a causal–contingent–common (CCC) pathway model (Kendler et al., Reference Kendler, Karkowski, Corey, Prescott and Neale1999a) that allows for the concurrent estimation of quantity conditional on drug use initiation as well as for estimating the relationship between drug use initiation and drug use quantity (see Figure 1). The CCC model estimates separate liabilities for drug use initiation and for quantity of drug use given initiation. Once adolescents initiate smoking, previous studies have found that heaviness of smoking (light, moderate, or heavy cigarette use; Huizink et al., Reference Huizink, Levälahti, Korhonen, Dick, Pulkkinen, Rose and Kaprio2010; Koopmans et al., Reference Koopmans, Slutske, Heath, Neale and Boomsma1999) and the presence of nicotine dependence as measured by the Fagerstrom Test for Nicotine Dependence (Haberstick et al., Reference Haberstick, Timberlake, Ehringer, Lessem, Hopfer, Smolen and Hewitt2007; Heatherton et al., Reference Heatherton, Kozlowski, Frecker and Fagerstrom1991; Vink et al., Reference Vink, Willemsen and Boomsma2005) are influenced by genetic factors, but no significant shared environmental effects have been reported. In a study of 11- to 19-year-old twins, the same additive genetic and shared environmental factors that influenced initiation of cigarette use were found to contribute to the variance in the number of cigarettes smoked (Fowler et al., Reference Fowler, Lifford, Shelton, Rice, Thapar, Neale and Van Den Bree2007). The present study extends previous research in this area (Do et al., Reference Do, Prom-Wormley, Eaves, Silberg, Miles and Maes2015; Öncel et al., Reference Öncel, Dick, Maes and Alıev2014) by characterizing in greater detail the developmental changes in the heritability of the quantity of cigarettes smoked per day conditional on cigarette use initiation across different age groups spanning adolescence and young adulthood and testing for age-specific sex differences among twins from a nationally representative US sample.
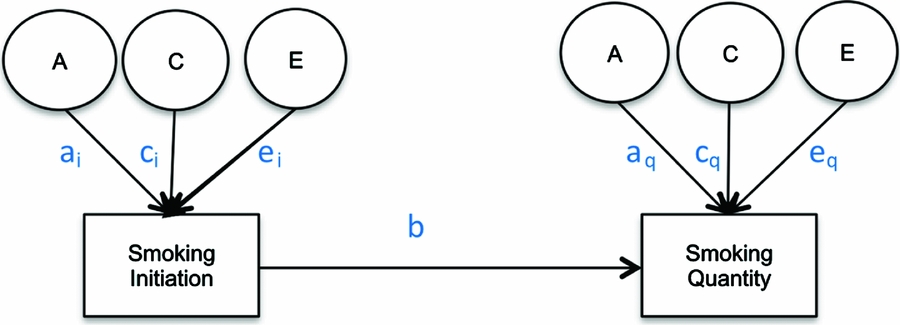
FIGURE 1 Causal–contingent–common pathway model.
Material and Methods
Sample
Data for this study came from twin pairs available in the National Longitudinal Study of Adolescent to Adult Health (Add Health; Harris et al., Reference Harris, Halpern, Smolen and Haberstick2006, Reference Harris, Halpern, Haberstick and Smolen2013). The Add Health study recruited adolescent participants when they were enrolled in high school and subsequently assessed them through three in-home interviews, for four waves of longitudinal data. In the first wave, participants’ age ranged from 12 to 17 years and in the last wave, participants ranged in age from 28 to 33 years. At each wave of the four-wave longitudinal study, participants reported on whether they had ever smoked a cigarette, and if they indicated they had smoked, they were subsequently asked how many cigarettes they had smoked per day in the previous 30 days.
Zygosity of the twin pairs was determined by matching on 12 molecular genetic markers and through self-report questions regarding the twins’ similarity (Harris et al., Reference Harris, Halpern, Smolen and Haberstick2006). For this study, participants were divided into five groups based on sex and zygosity (monozygotic males (MZM), monozygotic females (MZF), dizygotic males (DZM), dizygotic females (DZF), and opposite sex dizygotic twins (DZO) so that we could test for qualitative and quantitative sex differences in the sources of genetic and environmental influences on the phenotypes.
In the Add Health study, data were available for 740 twin pairs aged 12 to 33 years. At younger ages (12 and 13 years), the prevalence of smoking was very low and no participants smoked more than 11 cigarettes per day, so data for this age group was not used. Beginning with age 14, we collapsed across ages resulting in having an age group of adolescent twins who were between 14 and 15 years of age (n = 571 individuals), a group of 16- to 17-year-old participants (n = 776 individuals), a group of 18- to 25-year-old participants (n = 1,329 individuals), and a group of participants who were aged 26–33 years (n = 1,308 individuals). Table 1 presents demographic information, the prevalence of having ever smoked a cigarette, and the percentage of individuals in each of the three categories of cigarette smoking quantity for each age group. Tetrachoric and polychoric correlations for cigarette smoking initiation and cigarette smoking quantity are available in Tables 2 and 3 respectively.
TABLE 1 Demographic Characteristics by Age Group
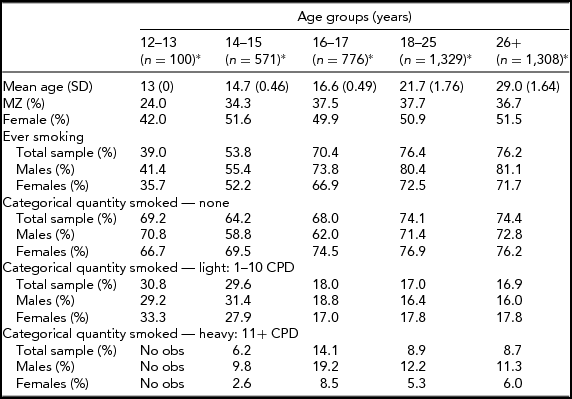
*n: number of individuals; obs: observations.
TABLE 2 Tetrachoric Correlations for Cigarette Use Initiation (95% CI) by Sex, Zygosity, and Age Group
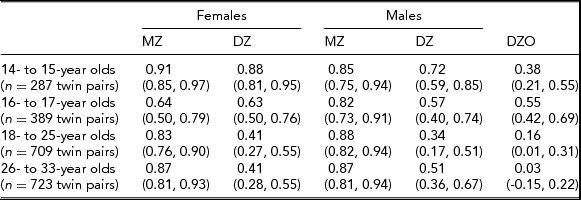
TABLE 3 Polychoric Correlations for Cigarette Quantity (95% CI) by Sex, Zygosity, and Age Group
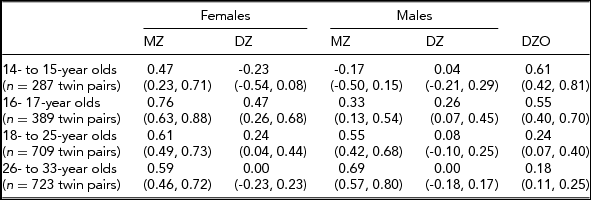
Non-smokers were not included in the correlations presented here for cigarette quantity. If the liabilities to cigarette quantity are independent of one another, then the twin correlations for cigarette quantity will accurately reflect the findings from the causal-contingent-common pathway model; otherwise, if the liabilities are dependent, they will not.
Measures
The aim of this study was to examine developmental changes in the etiology of smoking cigarettes, so cigarette smoking initiation and cigarette smoking quantity variables were created for each available age in the Add Health data. The smoking initiation variable was coded 1 when participants responded yes, or 0 when the answer was no to the survey question: ‘Have you ever smoked a cigarette?’ Subsequently, those participants who answered yes to having initiated smoking were asked how many cigarettes they had smoked in the previous 30 days. The answer to this question was used to create the smoking quantity variable. In Wave 3 of the Add Health data, the options available to participants to respond to the question about how many cigarettes they had smoked in the previous 30 days were ordinal categories rather than open-ended, as in waves 1, 2, and 4. Therefore, the quantity smoked variable used in the present analyses was an ordered categorical variable such that ‘0’ referred to not having smoked any cigarettes in the previous 30 days, ‘1’ referred to smoking between 1 and 11 cigarettes per day, and ‘2’ indicated smoking more than 11 cigarettes per day. Further, those participants who reported not having ever smoked a cigarette (the never smokers) were coded as having missing information for the smoking quantity variable as has been suggested by Neale and colleagues (Reference Neale, Harvey, Maes, Sullivan and Kendler2006).
Analysis Plan
Behavioral genetic studies are designed to uncover the sources of individual differences that give rise to variations in a phenotype and take advantage of the differences in the proportion of segregating genes between pairs of monozygotic (MZ) and dizygotic (DZ) twins who are reared together. MZ twins share 100% of their segregating genes, while DZ twins share on average 50% of their genes. If genetic factors contribute to a phenotype then MZ twins would be expected to be more similar than DZ twins. The variance in a phenotype is partitioned into additive genetic (A), shared environmental (C), or unique environmental (E) sources, where additive genetic sources of variance involve the cumulative effects of individual genes on a phenotype. Shared environmental sources are aspects of the environment that both twins experience and increase their similarity. Non-shared environmental sources of variance are aspects of the environment that contribute to making twins different in the same family. Measurement error is also contained in the non-shared environmental component.
The first step in data analysis involved testing the assumptions that the thresholds for cigarette smoking initiation were equal across twin order, sex, and zygosity for each age group. Then univariate analyses for binary variables decomposed the variance of cigarette smoking initiation into additive genetic (A), shared environmental (C), and unique environmental (E) sources and tested sex differences. Two types of sex differences were tested for: differences in the magnitude of genetic effects between males and females (quantitative sex differences) as well as differences due to a different set of genes influencing the phenotype in males than in females (qualitative sex differences). Quantitative and qualitative sex differences in cigarette smoking initiation were tested by comparing the likelihood statistic (-2LL) produced by reduced models with the likelihood of the full model. The difference in likelihood between the two models is known as the likelihood ratio test and is asymptotically distributed as χ2.
Causal–contingent–common analyses
Age-specific CCC models (Kendler et al., Reference Kendler, Karkowski, Corey, Prescott and Neale1999a; Maes et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004) for ordered categorical variables were run, starting with a full, sex-specific model where the three sources (A, C, E) of variance were included for both cigarette smoking initiation and cigarette quantity smoked, and included a common pathway between initiation and quantity (Model 1). First, an overall test for sex differences was performed (Model 2), followed by an overall test for the significance of genetic factors for both smoking initiation (ai in Figure 1) and smoking quantity (aq in Figure 1) at once (Model 3), and shared environmental contributions to smoking initiation and smoking quantity simultaneously (Model 4). The significance of the common pathway between smoking initiation and quantity smoked (b in Figure 1) was tested at each age group (Model 5). A common pathway coefficient of 0 would indicate that smoking initiation and quantity smoked are independent processes for that particular age group, while a common pathway coefficient of 1 would indicate that smoking quantity is on the same continuum as smoking initiation. The statistical significance of the variance components for smoking initiation (ai and ci in Figure 1) and for smoking quantity (aq and cq in Figure 1) was tested separately by fixing each to zero and examining model fit (Models 6–9). Analyses were done in R (RCore, Reference RCore2012) using the OpenMx package (Boker et al., Reference Boker, Neale, Maes, Wilde, Spiegel, Brick and Bates2011).
Results
Prevalence of lifetime cigarette use by age group is presented in Table 1. The prevalence rates for this sample are in line with the national estimates available for the years during which the survey was carried out (Johnston et al., Reference Johnston, O’Malley, Bachman and Schulenberg2013). Slightly over half of the 14- to 15-year olds, 70% of the 16- to 17-year olds, and over three-quarters of both the 18- to 25-year olds and 26- to 33-year olds had ever smoked a cigarette. Of the 14- to 15-year olds who had ever smoked, 29.6% were light smokers, and 6.2% were heavy smokers. The proportion of heavy smokers was highest in the 16- to 17-year olds (14.1%) and lowest in the 26- to 33-year old group (8.7%). Table 1 presents the prevalence of lifetime cigarette use and quantity of cigarettes smoked by sex and zygosity.
Age-Specific Univariate Models of Smoking Initiation
For each age group, we first fitted a saturated model, where the thresholds and correlations for cigarette smoking initiation were allowed to vary by twin order, sex, and zygosity. Then twin model assumptions were tested by evaluating changes in model fit when the thresholds were constrained across twin order, zygosity, same- and opposite-sex twins, and for males and females (results available upon request). Thresholds could be equated across twin order, zygosity (except at young adulthood), same- and opposite sex, and males and females (except in the oldest age group). Next, whether different genetic factors or environmental influences in males and females contribute to the variance in cigarette smoking initiation (qualitative sex differences), as well as whether males and females differed in the magnitude of additive genetic effects (quantitative sex differences), were tested for at each age group. There was no evidence of qualitative or quantitative sex differences in cigarette smoking initiation at any of the age groups (results available upon request).
Age-Specific Causal–Contingent–Common Pathway Models
For the 14- to 15-year-old group, the full CCC pathway model allowed the loadings of variance components for cigarette smoking initiation and quantity of cigarettes smoked to be freely estimated across sexes (Table 4, Model 1). Constraining the parameters to be equal across sexes did not cause the fit of the model to deteriorate (Model 2: χ2 = 7.52, df = 9, p = .583, see Table 3). Next, we tested the overall significance of the shared environment or the genetic contribution to liability of smoking initiation and smoking quantity, and found that excluding the shared environment significantly worsened the fit of the model (Model 3: χ2 = 6.87, df = 2, p = .032) but not when dropping the influence of additive genetic factors (Model 4: χ2 = 4.72, df = 2, p = .094). We then tested the significance of the beta coefficient between smoking initiation and smoking quantity and found that the common pathway in this age group was not significant (Model 5: χ2 = 2.54, df = 1, p = .111). Subsequent models for this age group did not include the common pathway between smoking initiation and smoking quantity. We then tested the significance of the additive genetic contribution to smoking initiation (Model 6) and smoking quantity (Model 7) and that of the shared environment on smoking initiation (Model 8) and smoking quantity (Model 9). We found that additive genetic and shared environmental influences were significant for smoking initiation (Model 6: χ2 = 4.58, df = 1, p = .032 and Model 8: χ2 = 6.15, df = 1, p = .013) but not for smoking quantity (Model 7: χ2 = 0.0, df = 1, p = 1.000 and Model 9: χ2 = 0.34, df = 1, p = .56).
TABLE 4 Causal–Contingent–Common Pathway Model Comparison by Age Group
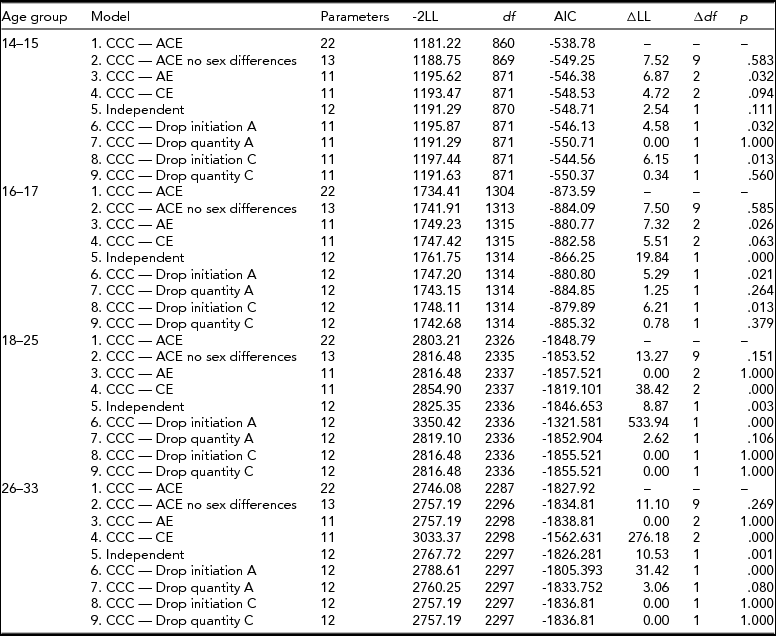
For the 16- to 17-year-old group, constraining the variance components and the common pathway between cigarette smoking initiation to quantity of cigarettes smoked to be equal across males and females did not worsen model fit (χ2 = 7.50, df = 9, p = .585). Removing the effects of the shared environment (Model 3: χ2 = 7.32, df = 2, p = .026) caused a significant reduction in the fit of the model but not when additive genetic effects were removed (Model 4: χ2 = 5.51, df = 2, p = .063). The significance of the common pathway between cigarette smoking initiation and quantity of cigarettes smoked was tested by setting it to zero and resulted in worse model fit (Model 5: χ2 = 19.84, df = 1, p < .0001), suggesting that for the 16- to 17-year-old group, the liabilities to smoking initiation and smoking quantity were correlated. Dropping either additive genetic effects from smoking initiation (Model 6: χ2 = 5.29, df = 1, p = .021) or shared environmental contributions to smoking initiation (Model 8: χ2 = 6.21, df = 1, p = .013) worsened the fit of the model, but removing the influence of additive genetic effects (Model 7: χ2 = 1.25, df = 1, p = .264) or shared environmental influences from smoking quantity (Model 9: χ2 = 0.78, df = 1, p = .379) did not.
For the 18- to 25-year olds, testing a model where the estimates in the parameters were equated across males and females resulted in no significant reduction in model fit (Model 2: χ2 = 13.27, df = 9, p = .151), so the subsequent models contained equal A, C, and E estimates and the common pathway across sex. Next, a model that tested the overall contribution of the shared environment was not significant (Model 3: χ2 = 0.0, df = 2, p = 1.0), but that of additive genetic effects was significant (Model 4: χ2 = 38.42, df = 2, p < .001). Next, whether the liabilities to smoking initiation and smoking quantity were independent of one another was tested by removing the common pathway from the model and resulted in a worse fit (Model 5: χ2 = 8.87, df = 1, p < .01). In subsequent models for this age group, the common pathway was included. The specific influence of additive genetic effects was found to be important for smoking initiation (Model 6: χ2 = 534.23, df = 1, p < .001) but not for smoking quantity (Model 7: χ2 = 2.62, df = 1, p = .106). The influence of the shared environment did not deteriorate model fit on liability to smoking initiation (Model 8: χ2 = 0.0, df = 1, p = 1.0), or smoking quantity (Model 9: χ2 = 0.0, df = 1, p = 1.0).
Lastly, for the 26- to 33-year-old age group, equating the estimates across sex did not result in worse model fit (Model 2: χ2 = 11.1, df = 9, p = .269), suggesting that for this age group there were no significant differences by sex in the estimates for A, C, and E or in the beta coefficient of the common pathway. Removing the overall influence of the shared environment did not cause a reduction in model fit (Model 3: χ2 = 0.0, df = 2, p = 1.0), but the removal of additive genetic influences did cause the model to fit worse (Model 4: χ2 = 276.18, df = 2, p < .001). When the common pathway between smoking initiation and smoking quantity was removed in Model 5, the model fit worsened (χ2 = 10.53, df = 1, p < .01), suggesting that the liabilities to smoking initiation and smoking quantity are correlated at this age. Additive genetic influences were important for smoking initiation (Model 6: χ2 = 460.24, df = 1, p < .001) but not for smoking quantity (Model 7: χ2 = 3.06, df = 1, p = .08). The influence of shared environment was not significant for smoking initiation or smoking quantity (Model 8: χ2 = 0.0, df = 1, p = 1.0; Model 9: χ2 = 0.0, df = 1, p = 1.0).
The variance component estimates and associated 95% confidence intervals (CI) from the CCC pathway model without sex differences are presented in Table 5 for each age group. The influence of risk factors for quantity of cigarettes smoked that exert their influence through their effect on smoking initiation was found to increase through adolescence and into young adulthood. For the 14- to 15-year-old age group, we found that risk factors influencing smoking initiation were independent of those that contributed to smoking quantity. In the three older age groups, the risk factors predisposing to smoking initiation were correlated with those that influence smoking quantity (98%, 62%, and 65%, respectively) suggesting substantial overlap in the factors that influence initiating cigarette use and the number of cigarettes smoked among older adolescents and young adults.
TABLE 5 Parameter Estimates (95% CI) From CCC Pathway No Sex Differences Model
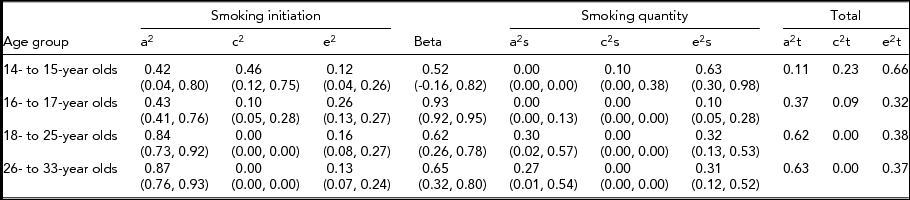
We present estimates of the variance components for the same model across all age groups (Table 5), even though the causal path was not significant for age group 14–15 and some of the genetic and shared environmental contributions were not significant. We estimated that for the 14- to 15-year-old age group, 42% of the variance in liability to smoking initiation was accounted for by additive genetic factors, 46% by shared environmental factors, and 12% by non-shared environmental factors. Only non-shared environmental factors accounted for a significant portion of the variance (63%) in quantity of cigarettes smoked. In the 16- to 17-year-olds, 43% of the variance in liability to smoking initiation was accounted for by additive genetic factors, 10% was attributable to the shared environment, and 26% by non-shared environment. The additive genetic and shared environmental influences on smoking initiation were found to be almost completely shared with those that influence smoking quantity (causal path estimated at 0.93) but no additional genetic effects or shared environmental effects were found for this age group to influence the quantity of cigarettes smoked. The estimates for the 18- to 25-year-old and the 26- to 33-year-old age groups indicated that only additive genetic factors accounted for familial resemblance in liability to smoking initiation (84% and 87% respectively), and that these factors influenced smoking quantity through a substantial common path. Additional genetic effects were found to influence the quantity of cigarettes smoked for both young adult age groups (about 30% each).
Discussion
The aim of the present study was to examine developmental changes in the etiology of smoking behavior by modeling the influence of additive genetic and shared environmental influences of quantity of cigarettes smoked conditional on having initiated cigarette smoking. Consistent with previous work, the results of the present study did not find sex differences in the proportion of variance explained by genetic or environmental factors in adolescent smoking initiation or quantity of cigarettes smoked (Boomsma et al., Reference Boomsma, Koopmans, Doornen and Orlebeke1994; McGue et al., Reference McGue, Elkins and Iacono2000; Rende et al., Reference Rende, Slomkowski, McCaffery, Lloyd-Richardson and Niaura2005).
The results indicate the presence of developmental changes in the degree of overlap between the liability of smoking initiation and smoking quantity from adolescence and into young adulthood. The common pathway between liability to cigarette smoking initiation and cigarette smoking quantity could be dropped from the model in early adolescence, suggesting that different risk factors influence whether individuals have initiated cigarette use and how many cigarettes they subsequently smoked. For older adolescents and young adults, removing the common pathway between liability to initiate smoking and quantity smoked worsened model fit, suggesting the presence of correlated liabilities between cigarette use initiation and cigarette smoking quantity, as has been found in previous adult twin studies (Boomsma et al., Reference Boomsma, Koopmans, Doornen and Orlebeke1994; Maes et al., Reference Maes, Sullivan, Bulik, Neale, Prescott, Eaves and Kendler2004). Thus, with age, the factors that influence the initiation of smoking also play a role in how many cigarettes individuals smoke. At ages 16–17, we found that 93% of the variance in liability to the amount of cigarette smoked was shared with the liability to initiate cigarette smoking. The corresponding proportions of variance in liability of cigarette smoking quantity shared with smoking initiation are 62% for the 18- to 25-year-old group, and 65% for the 26- to 33-year-old group. Recent reports of adolescent and young adult smoking have shown similar estimates for the proportion of variance in liability shared between smoking initiation and quantity (Do et al., Reference Do, Prom-Wormley, Eaves, Silberg, Miles and Maes2015; Öncel et al., Reference Öncel, Dick, Maes and Alıev2014).
Further, the findings suggest developmental changes in the factors that contribute to smoking initiation and number of cigarettes smoked when quantity smoked is modeled contingent on initiation. For the youngest age group, additive genetic and shared environmental factors were found to contribute to the liability of smoking initiation, but there were no new additive genetic or shared environmental influences specific to the liability of smoking quantity. Previous adolescent and young adult twin studies that have estimated the heritability of cigarettes smoked contingent on cigarette use initiation have included twins of a broader age range (Boomsma et al., Reference Boomsma, Koopmans, Doornen and Orlebeke1994; Öncel et al., Reference Öncel, Dick, Maes and Alıev2014) during a time when the etiology of smoking behavior is changing rapidly. The differences in the results observed in the present study compared with previous findings may be due to our age-specific focus, which uncovered a developmental change that may have been previously overlooked. Consistent with our findings, recently, Do and colleagues (Reference Do, Prom-Wormley, Eaves, Silberg, Miles and Maes2015) reported no evidence of new additive genetic effects on the quantity of cigarettes smoked in early adolescence. As individuals aged into late adolescence and young adulthood, the influence of additive genetic factors became more important for the initiation of cigarette use, a finding that is consistent with previous research (Do et al., Reference Do, Prom-Wormley, Eaves, Silberg, Miles and Maes2015; Kendler et al., Reference Kendler, Chen, Dick, Maes, Gillespie, Neale and Riley2012; Koopmans et al., Reference Koopmans, Doornen and Boomsma1997). For the two oldest age groups (18–25 and 26–33), shared environmental influences did not contribute significantly to the liability to initiate smoking but additive genetic effects were significant.
We show that there are changes in the overlap of additive genetic and environmental factors that play a role in the initiation of smoking during early adolescence and into young adulthood. This finding adds to the literature on the developmental changes in the contributions of additive genetic and shared environmental factors on smoking initiation (Boomsma et al., Reference Boomsma, Koopmans, Doornen and Orlebeke1994; Han et al., Reference Han, McGue and Iacono1999) and smoking quantity and delineates the developmental shift from shared environmental influences on smoking initiation to additive genetic ones (Do et al., Reference Do, Prom-Wormley, Eaves, Silberg, Miles and Maes2015; Koopmans et al., Reference Koopmans, Doornen and Boomsma1997; Öncel et al., Reference Öncel, Dick, Maes and Alıev2014).
It has been known that smoking behaviors are complex behavioral traits that are under genetic and environmental influence (Kendler et al., Reference Kendler, Chen, Dick, Maes, Gillespie, Neale and Riley2012; Munafo et al., Reference Munafo, Clark, Johnstone, Murphy and Walton2004). The present study adds to a growing body of literature (Do et al., Reference Do, Prom-Wormley, Eaves, Silberg, Miles and Maes2015; Öncel et al., Reference Öncel, Dick, Maes and Alıev2014) indicating that the genetic and environmental risk factors for smoking behaviors change over adolescence and into young adulthood. Efforts to find the gene clusters associated with cigarette use have resulted in identifying dopamine receptor genes associated with cigarette use initiation, progression into regular use, and nicotine dependence (Munafo et al., Reference Munafo, Clark, Johnstone, Murphy and Walton2004). Further, once individuals begin using cigarettes and are exposed to nicotine, nicotinic acetylcholine receptor genes have been found to be associated with both the number of cigarettes smoked per day (Liu et al., Reference Liu, Tozzi, Waterworth, Pillai, Muglia, Middleton and Waeber2010; Saccone et al., Reference Saccone, Culverhouse, Schwantes-An, Cannon, Chen, Cichon and Keskitalo-Vuokko2010; Tobacco & Genetics Consortium, 2010) and nicotine dependence (Chen et al., Reference Chen, Chen, Williamson, An, Hettema, Aggen and Kendler2009; Wang et al., Reference Wang, van der Vaart, Xu, Seneviratne, Pomerleau, Pomerleau and Li2014; Ware et al., Reference Ware, van den Bree and Munafò2011). However, this work has focused almost exclusively on adult samples. Whether the same genes are important for younger samples has yet to be determined. Our results indicate that during early adolescence, the shared environment plays a substantial role in cigarette use, thus characterizing how different environmental exposures interact with gene clusters in increasing risk for initiating and maintaining cigarette use during this age group will provide a comprehensive understanding of the unfolding of cigarette use. The implications of these findings for the future gene finding research suggest the need to search for genetic variants for cigarette use initiation and quantity that are developmentally specific.
Limitations
The findings of the present study should be considered with the following limitations in mind. First, to examine developmental changes, the sample of available twins in the data was divided into groups according to age, and this may have reduced the power to detect significant sex effects, especially at the youngest ages where the sample of twin pairs was 287. Second, to have a comparable quantity smoked variable across each assessment, we turned a continuous measure of cigarettes smoked per day into an ordinal variable, which may have resulted in loss of information to detect individual differences. Lastly, recent research has indicated important racial/ethnic differences in age of smoking onset (Clark et al., Reference Clark, Doyle and Clincy2013), in quantity of cigarettes smoked (Gutman et al., Reference Gutman, Eccles, Peck and Malanchuk2011), and in the development of nicotine dependence (Duncan et al., Reference Duncan, Lessov-Schlaggar, Sartor and Bucholz2012). In the present analyses, we did not test for the possibility that additive genetic influences on the liability to initiate smoking and on the quantity of cigarettes smoked would differ by racial/ethnic background.
Acknowledgments
The research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award numbers R01DA025109 (H.H.M.) and K01DA036681 (C.B.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.








