Introduction
Anti-Müllerian hormone (AMH) is a peptide growth factor and a member of the tissue growth factor beta superfamily that is produced after 36 weeks of gestation by the granulosa cells of primary, secondary, pre-antral and early antral follicles in female fetuses (Durlinger et al., Reference Durlinger, Kramer, Karels, de Jong, Uilenbroek, Grootegoed and Themmen1999; Rajpert-De Meyts et al., Reference Rajpert-De Meyts, Jørgensen, Graem, Müller, Cate and Skakkebaek1999; Weenen et al., Reference Weenen, Laven, Von Bergh, Cranfield, Groome, Visser, Kramer, Fauser and Themmen2004). AMH peaks after puberty (Rajpert-De Meyts et al., Reference Rajpert-De Meyts, Jørgensen, Graem, Müller, Cate and Skakkebaek1999) and may participate in regulating the recruitment of the antral follicular cohort (reviewed in Baerwald et al., Reference Baerwald, Adams and Pierson2012).
There is a natural decline in AMH levels with age, culminating in undetectable levels after menopause, therefore AMH is highly correlated with baseline antral follicle count (Fanchin et al., Reference Fanchin, Schonäuer, Righini, Guibourdenche, Frydman and Taieb2003) and ovarian ageing (de Vet et al., Reference de Vet, Laven, de Jong, Themmen and Fauser2002), and considered the most valuable marker of ovarian reserve (van Rooij et al., Reference van Rooij, Broekmans, te Velde, Fauser, Bancsi, de Jong and Themmen2002; Eldar-Geva et al., Reference Eldar-Geva, Ben-Chetrit, Spitz, Rabinowitz, Markowitz, Mimoni, Gal, Zylber-Haran and Margalioth2005; Tremellen et al., Reference Tremellen, Kolo, Gilmore and Lekamge2005; Nakhuda et al., Reference Nakhuda, Chu, Wang, Sauer and Lobo2006; Nakhuda et al., Reference Nakhuda, Sauer, Wang, Ferin and Lobo2007; Nardo et al., Reference Nardo, Gelbaya, Wilkinson, Roberts, Yates, Pemberton and Laing2009).
Although the AMH level can be used as a predictor of the response to controlled ovarian stimulation (COS), contradictory reports have arisen concerning its influence on oocyte quality. Borges et al. (Reference Borges, Braga, Setti, Figueira and Iaconelli2017) have hypothesized that lower AMH concentrations would affect oocyte and embryo development and demonstrated that AMH levels were positively correlated with the quality of the oocyte, but with neither the embryo quality on the cleavage stage nor the blastocyst development chance. Conversely, Loh and Maheshwari (Reference Loh and Maheshwari2011) and Hazout et al. (Reference Hazout, Cohen Bacrie, Mendoza, Mendoza and Tesarik2005) observed that the AMH level was not predictive of oocyte quality. Silberstein et al. (Reference Silberstein, MacLaughlin, Shai, Trimarchi, Lambert-Messerlian, Seifer, Keefe and Blazar2006) showed that AMH levels were correlated with embryo quality, however, in several studies, no significant effect was found (Hazout et al., Reference Hazout, Cohen Bacrie, Mendoza, Mendoza and Tesarik2005; Smeenk et al., Reference Smeenk, Sweep, Zielhuis, Kremer, Thomas and Braat2007; Lie Fong et al., Reference Lie Fong, Baart, Martini, Schipper, Visser, Themmen, de Jong, Fauser and Laven2008).
The use of time-lapse imaging (TLI) technology has allowed the assessment of morphological changes with the exact time point of occurrence, the possibility of assessing complete embryonic development, and the ability to investigate parameters that are potentially associated with embryo morphokinetic development. Therefore, it may be a valuable tool to evaluate the correlation between serum AMH concentration and the competence of both the oocyte and developing embryo. A previous study failed to demonstrate a significant association between serum AMH and KIDScore embryo quality generated by TLI (Bhide et al., Reference Bhide, Escriba, Srikantharajah, Joshi, Gudi, Shah, Acharya and Homburg2017) in in vitro fertilization (IVF) patients when embryo quality was assessed using KIDScores.
Embryos from the same patient or cycle share a similar pattern of behaviour, called clustering, which must be considered when the statistical analysis has the embryo as its base unit. However, this is mostly inadequately considered in most studies, including the aforementioned study, which can lead to invalid conclusions (Wampold and Serlin, Reference Wampold and Serlin2000).
In this study, we hypothesized that serum AMH concentrations may correlate with the speed and pattern of cell divisions, something that would not be detected by conventional morphological embryo assessment. The aim of this study was to investigate the relationship between serum AMH levels and morphokinetic parameters of embryos derived from ICSI cycles, considering clustering of data (multiple embryos per patient).
Materials and methods
Patients and experimental design
This historical cohort study was performed in a private university-affiliated IVF centre, between March 2019 and December 2020. Kinetic data were analyzed in 902 embryos individually cultured in a TLI incubator (EmbryoScope+, Vitrolife A/S, Viby J, Denmark) until day 5 of development, deriving from 114 patients undergoing their first ICSI cycle. The time of specific events from the point of insemination was determined using TLI. Serum AMH assays were included as a standard measure in the IVF programme. Blood samples were collected in the morning of days 2–5 of the menstrual cycle, no earlier than 3 months prior to the start of each cycle and AMH was measured using the Elecsys AMH Plus assay (Roche Diagnostics, Meylan France). The effects of serum AMH concentrations on morphokinetic events and ICSI outcomes were investigated considering clustering of data (multiple embryos per cycle), using generalized mixed models. All patients signed a written informed consent form in which they agreed to share the outcomes of their cycles for research purposes, and the study was approved by the local Institutional Review Board.
Controlled ovarian stimulation and laboratory procedures
On the third day of the cycle, COS was started by the administration of r-FSH (300IU follitropin alpha, Gonal-F, Serono, Geneva, Switzerland or 16 μg follitropin delta, Rekovelle®, Ferring, Saint-Prex, Switzerland) daily doses. r-FSH dose was adjusted according to follicular development, which was monitored using an ultrasound scan.
When at least one follicle ≥14 mm was visualized, a pituitary blockage was performed using gonadotropin-releasing hormone (GnRH) antagonist (GnRHa, Cetrotide®; Merck KGaA, Darmstadt, Germany). When three or more follicles attained a mean diameter of ≥17 mm and adequate serum estradiol levels were observed, r-FSH and GnRH antagonist administrations were stopped, and final follicular maturation was triggered by the administration of recombinant human chorionic gonadotropin (r-hCG, 250 µg, Ovidrel®, Merck KGaA, Geneva, Switzerland). Oocyte retrieval was performed transvaginally hours later. After denudation for the removal of cumulus–oocyte complex cells, mature oocytes in metaphase II were selected for ICSI.
Semen analysis and preparation
Semen samples were collected in the laboratory. After liquefaction for 30 min, sperm count and motility assessment were performed by following the instructions of the count chamber manufacturer (Leja® slide, Gynotec Malden, Nieuw-Vennep, The Netherlands). Sperm samples were prepared using a two-layered density gradient centrifugation technique (50% and 90% Isolate, Irvine Scientific, Santa Ana, CA, USA).
Intracytoplasmic sperm injection
Intracytoplasmic sperm injection was performed according to Palermo et al. (Reference Palermo, Joris, Devroey and Van Steirteghem1992). Sperm was selected at ×400 magnification using an inverted Nikon Eclipse TE 300 microscope and injected into the oocytes in a micro-injection dish prepared with buffered medium (Global w/HEPES, LifeGlobal, Guilford, USA) covered with paraffin oil (Paraffin oil P.G., LifeGlobal), on an inverted microscope heated stage (37°C ± 0.5°C).
Embryo culture
Injected oocytes were cultured individually in a 16-well culture dish (Embryoslide, Unisense Fertilitech, Aarhus, Denmark), in 360 μl of continuous single-culture medium (Global® total®, LifeGlobal), overlaid with 1.8 ml of mineral oil (Paraffin oil P.G., LifeGlobal) in a TL-monitored incubator (EmbryoScope+, Vitrolife) set at 37°C with an atmosphere of 6% O2 and 7.2% CO2 until day 5 of embryo development. The incubator high-definition camera was set up to record embryos’ images, in 11 focal planes, every 10 min. Recorded kinetic markers were timed to pronuclei appearance (tPNa, time point that the first PN is visible) and fading (tPNf, time point when PNs are no longer visible), completion of division to 2-cell-stage up to 8-cell-stage embryos: timed to two (t2), three (t3), four (t4), five (t5), six (t6), seven (t7), and eight cells (t8); and timed to the start of blastulation (tSB, time point that formation of blastocoel cavity is initiated through cavitation) and to blastulation (tB, time point that formation of blastocyst is complete through differentiation of inner cell mass and trophectoderm). Durations of the second (cc2, t3 − t2, cleavage from two to three cells) and third cell cycles (cc3, t5 − t3, cleavage from three to five cells) and time to complete synchronous divisions t2 − tPNf (s1, duration of cell division from PN fading to two cells), t4 − t3 (s2, duration of cells division from three to four cells) and t8 − t5 (s3, duration of cell division from five to eight cells) were calculated (Findikli and Oral, Reference Findikli and Oral2014). Data generated from EmbryoScope+ was analyzed using the EmbryoViewer software (Vitrolife, Viby J, Denmark). The incidences of multinucleation (presence of more than one nuclei in at least one blastomere) at the 2-cell and 4-cell stages and of abnormal cleavage patterns (direct cleavage, i.e. from one to three cells, or reverse cleavage, i.e. from three to two cells) were recorded for each embryo. Embryo annotations were performed by two senior embryologists and reviewed by the head embryologist.
Clinical follow-up
Embryo transfer was performed on day 5 of embryo development and one or two embryos were transferred per patient depending on embryo quality and maternal age. Embryo selection for transfer was based on the KIDScore algorithm.
Women with a positive pregnancy test, performed 10 days post embryo transfer, had a transvaginal ultrasound scan 2 weeks later. The clinical pregnancy was diagnosed upon detection of a fetal heartbeat. The pregnancy rate was calculated per embryo transfer. The implantation rate was the number of gestational sacs with fetal heartbeats divided by the number of transferred embryos. Miscarriage was defined as clinical pregnancy loss before 20 weeks.
Data analysis and statistics
Serum AMH concentration was treated as a continuous variable. Generalized mixed models (GMM) adjusted for potential confounders were used to study its effect on embryo morphokinetics. The models were generated using AMH as an independent variable and kinetic markers as dependent variables. Maternal and paternal ages, the total dose of FSH administered for COS and the number of retrieved oocytes were included as covariates in all models to control for their influence. A random effect was added to account for the correlation between the embryos within the same cycle, with linear distribution for morphokinetic data in hours (h) and known implantation diagnosis score (KIDScore) ranking, and binomial distribution for the incidences of abnormal cleavage patterns and multinucleation. For clinical outcomes, which were based on a single observation per couple, regression models (generalized linear models) were used without random effects, with linear distribution for implantation rate and binomial distribution for clinical pregnancy and miscarriage rates.
Regression models’ results were expressed as odds ratio (OR) or beta coefficient (B) with 95% confidence interval (CI) and P-values. A P-value < 0.05 was considered statistically significant. Data analysis was conducted using the Statistical Package for the Social Sciences (SPSS) 21 (IBM, New York, NY, USA).
Results
Demographic data concerning male and female partners, and the outcomes of ICSI cycles are shown in Table 1. Embryo morphokinetic development is shown in Table 2.
Table 1. Demographic characteristics and ICSI outcomes in study group (n = 114 cycles)
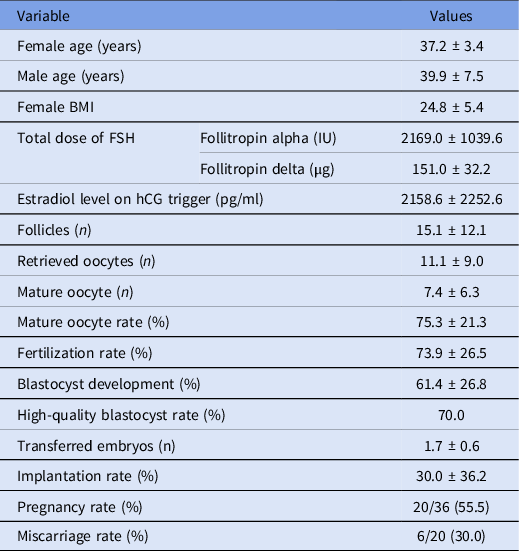
Note: Values are means ± standard deviation, unless otherwise noted. BMI, body mass index; FSH, follicle stimulating hormone; hCG, human chorionic gonadotropin; ICSI, intracytoplasmic sperm injection.
Table 2. Embryonic morphokinetic parameters in study group (n = 114 cycles and 902 embryos)

Values are mean ± standard deviation (SD); unless otherwise noted. h, hours; tPNa, time to pronuclei appearance; tPNf, time to pronuclei fading; t2, time to two cells; t3, time to three cells; t4, time to four cells; t5, time to five cells; t6, time to six cells; t7, time to seven cells; t8, time to eight cells; tSB, time to start blastulation; tB, time to blastulation; s1, time to complete t2 − tPNf synchronous divisions; s2, time to complete t4 − t3 synchronous divisions; s3, time to complete t8 − t5 synchronous divisions; cc2, duration of the second cell cycle (t3 − t2); cc3, duration of third cell cycle (t5 − t3).
The mean serum AMH concentration level was 2.24 ng/ml ± 3.13 ng/ml (median 1.36 ng/ml). The AMH impacted embryo morphokinetics nearly as a whole. Negative relationships were observed between AMH concentration levels and tPNf, t3, t4, t5, t6, t7, t8, tB, cc2, and s3 (Table 3). There was also a trend towards a positive correlation between AMH concentration and faster t2. Beta coefficients indicating the degree of change in the outcome variable for every one unit of change in the predictor variable, demonstrated that, for every unit increase in the AMH concentration level, there were decreases in tPNf of 0.047 h, in t3 of 0.070 h, in t4 of 0.080 h, in t5 of 0.075 h, in t6 of 0.105 h, in t7 of 0.120 h, in t8 of 0.170 h, in tB of 0.153 h, in cc2 of 0.041 h and in s3 of 0.086 h, meaning that t8 was the parameter most impacted by AMH levels, followed by tB (Figure 1).
Table 3. Results from regression analysis for the influence of serum AMH concentration levels on embryo morphokinetics (n = 114 cycles and 902 embryos)
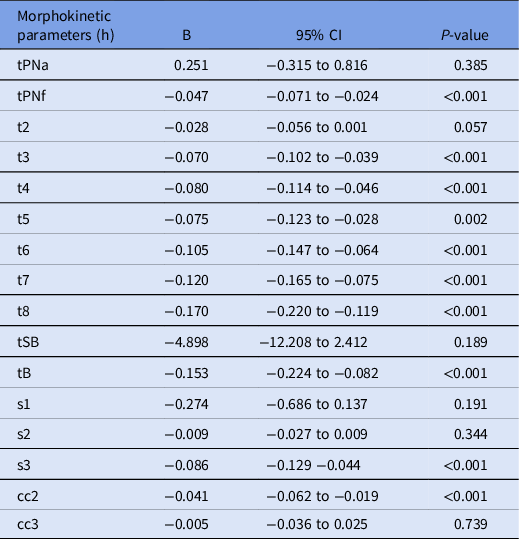
Note: Values are means ± standard deviation, unless otherwise noted. AMH, Anti-Müllerian hormone; h, hours; B, Beta coefficient; CI, confidence interval; tPNa, time to pronuclei appearance; tPNf, time to pronuclei fading; t2, time to two cells; t3, time to three cells; t4, time to four cells; t5, time to five cells; t6, time to six cells; t7, time to seven cells; t8, time to eight cells; tSB, time to start blastulation; tB, time to blastulation; s1, time to complete t2 − tPNf synchronous divisions; s2, time to complete t4 − t3 synchronous divisions; s3, time to complete t8 − t5 synchronous divisions; cc2, duration of the second cell cycle (t3 − t2); cc3, duration of third cell cycle (t5 − t3).

Figure 1. Illustration of faster embryo morphokinetic development according to increased serum AMH concentration levels. Note: AMH, anti-Müllerian hormone; tPNf, time to pronuclei fading; t3, time to three cells; t4, time to four cells; t5, time to five cells; t6, time to six cells; t7, time to seven cells; t8, time to eight cells; tB, time to blastulation (tB); s3, time to complete t8 − t5 synchronous division; cc2, duration of the of the second cell cycle (t3 − t2).
Neither the incidence of abnormal cleavage patterns (reverse or direct cleavages) (OR: 0.998, CI: 0.985–1.006) nor the incidences of multinucleation at 2-cell (OR: 0.811, CI: 0.585–1.126) and 4-cell stages (OR: 0.687, CI: 0.424–1.114) were correlated with the AMH concentration. The AMH concentration levels were positively correlated with the KIDScore ranking (Figure 2) and with the implantation rate (Figure 3). There was a trend towards a positive association with clinical pregnancy outcome (OR: 3.390, CI: 0.826–13.924; P = 0.090), but statistical significance was not reached, possibly due to the low number of transferred cycles. The miscarriage rate was not impacted by AMH (OR: 0.789, CI: 0.198–3.141).
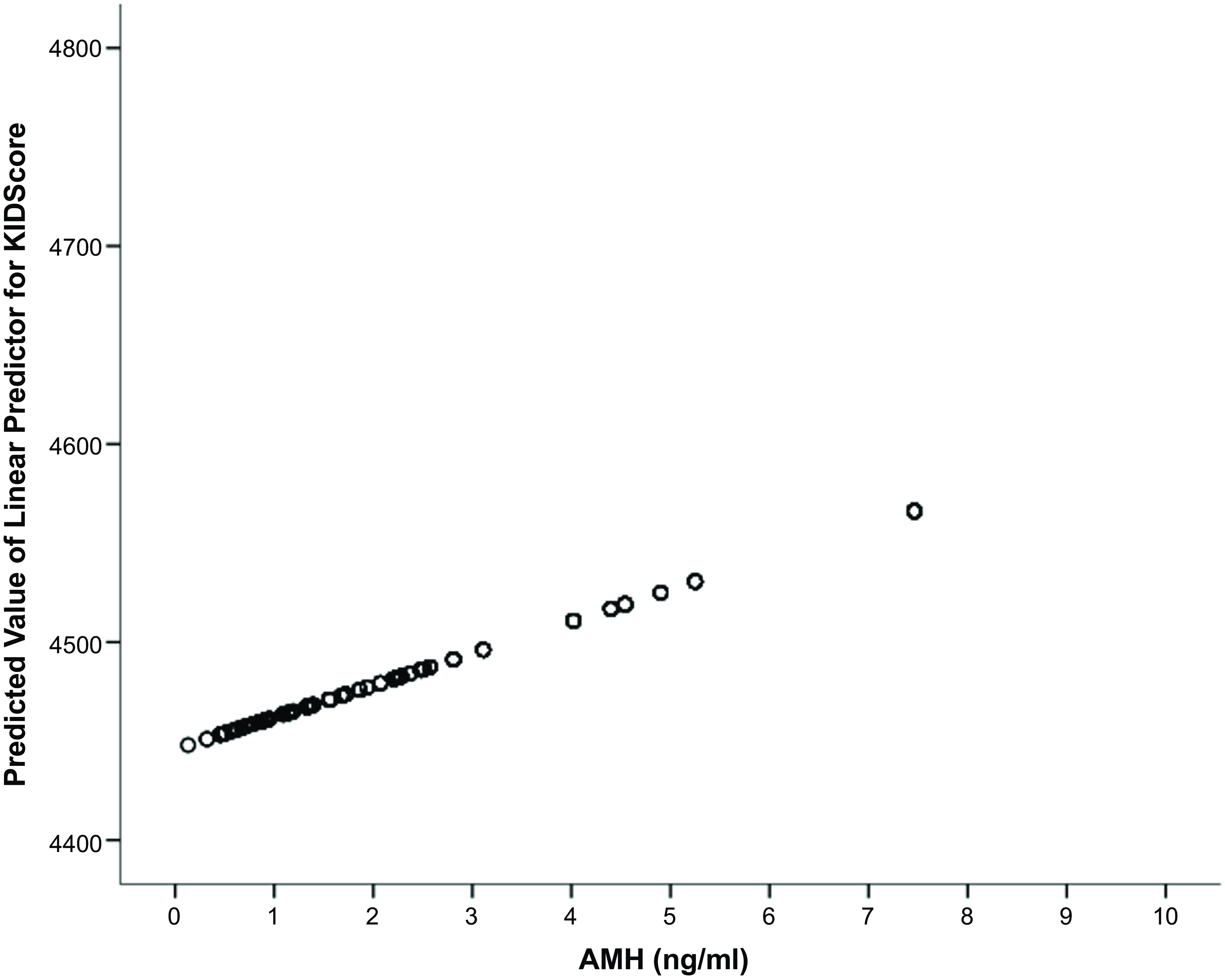
Figure 2. Illustration of the predictive value of AMH on embryos’ KIDScore ranking.
Note: AMH, anti-Müllerian hormone; KIDScore, known implantation diagnosis score.
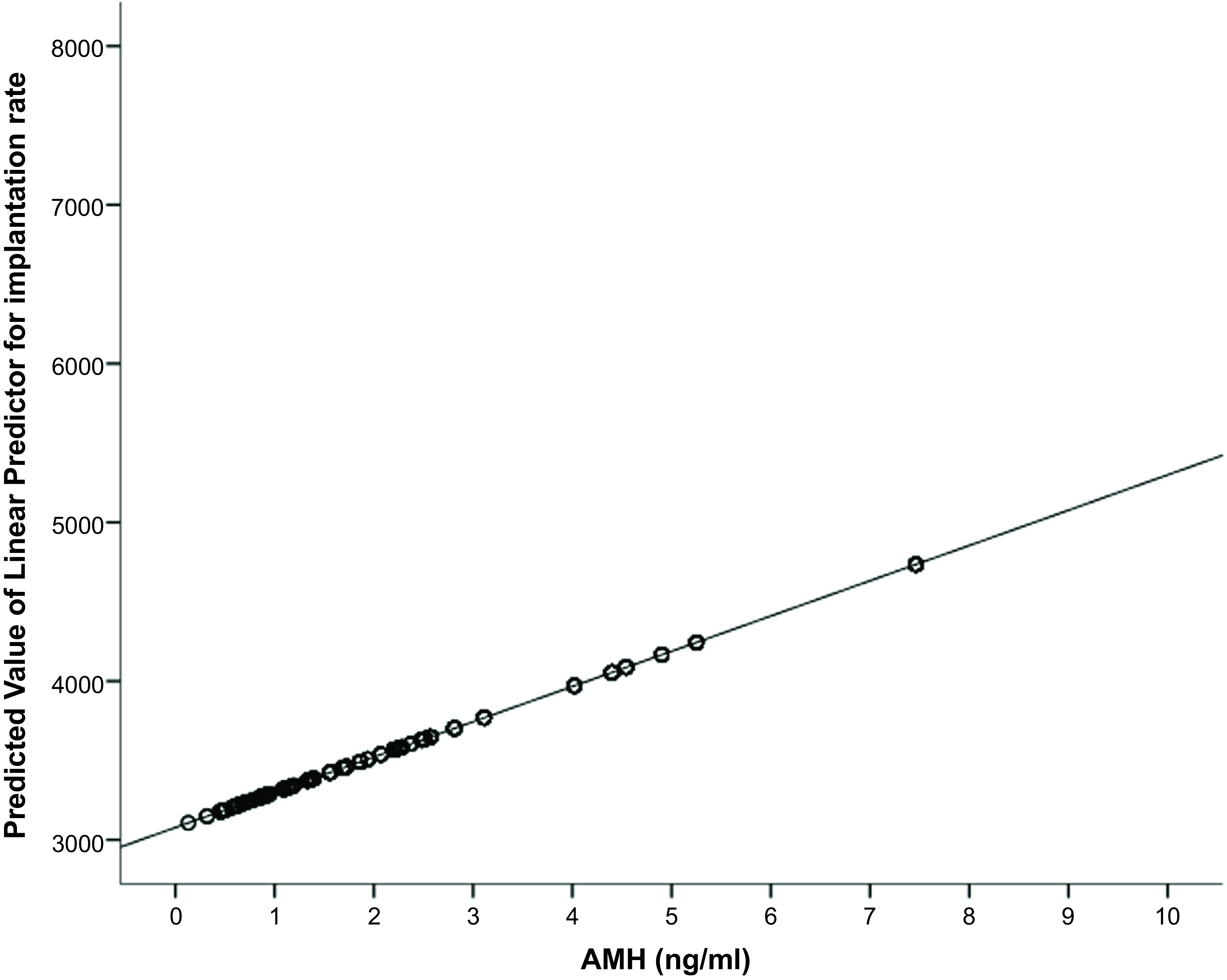
Figure 3. Illustration of the predictive value of AMH on embryos’ implantation rate.
Note: AMH, anti-Müllerian hormone.
Discussion
In the present study, we hypothesized that serum AMH concentration levels could have an effect on the speed and pattern of embryonic cell divisions. Our results demonstrated that increasing AMH levels positively impacted embryo quality and morphokinetics by decreasing the time to achieve several of the investigated kinetic events. Positive correlations between AMH concentration levels and KIDScore ranking, as well as for the implantation rate, were also noted.
These findings are of importance because it was previously demonstrated that delayed morphokinetic development may correlate with ICSI outcomes. We were able to detect correlations between AMH concentration levels as early as tPNf, which progressed to a cumulative earlier development until tB. Interestingly, it was previously demonstrated that embryos with high blastulation and implantation potential cleave from the 2-cell stage to the 8-cell stage progress earlier than those with low potential (Meseguer et al., Reference Meseguer, Herrero, Tejera, Hilligsøe, Ramsing and Remohí2011; Dal Canto et al., Reference Dal Canto, Coticchio, Mignini Renzini, De Ponti, Novara, Brambillasca, Comi and Fadini2012; Kirkegaard et al., Reference Kirkegaard, Kesmodel, Hindkjær and Ingerslev2013). Also, a shorter t4 was correlated previously with euploidy (Minasi et al., Reference Minasi, Colasante, Riccio, Ruberti, Casciani, Scarselli, Spinella, Fiorentino, Varricchio and Greco2016).
In the present study, despite the positive correlations that have been found between serum AMH concentration levels and faster developmental kinetics and implantation potential, its effect on clinical pregnancy outcome did not reach statistical significance, only a trend was observed. We suggest that this might have happened because clinical outcomes are associated with smaller sample sizes (only one observation per patient). Alternatively, morphokinetic variables and implantation follow linear and Poisson distributions, respectively, while pregnancy rate follows a binomial distribution, which is also interpreted differently in the statistical analysis. Finally, the KIDScore assessment may have improved the selection of embryos for transfer and therefore the pregnancy rate. However, this was out of the scope of this study and further studies are necessary to corroborate this theory.
The scientific basis for the relationship between serum AMH concentrations and speed up embryo morphokinetic development is unknown. However we could suggest that higher levels of AMH are associated with the retrieval of better-quality oocytes, which in turn results in better embryonic development. This could be explained by either an increased secretion of AMH by granulosa cells in poor quality oocytes or a potential positive effect of higher AMH levels on oocyte quality. It has been demonstrated previously that serum AMH levels are positively correlated with oocyte quality (Ebner et al., Reference Ebner, Sommergruber, Moser, Shebl, Schreier-Lechner and Tews2006; Irez et al., Reference Irez, Ocal, Guralp, Cetin, Aydogan and Sahmay2011), fertilization rate (Lekamge et al., Reference Lekamge, Barry, Kolo, Lane, Gilchrist and Tremellen2007; Majumder et al., Reference Majumder, Gelbaya, Laing and Nardo2010), embryo morphology (Silberstein et al., Reference Silberstein, MacLaughlin, Shai, Trimarchi, Lambert-Messerlian, Seifer, Keefe and Blazar2006; Lin et al., Reference Lin, Yao, Zhang, Zhang, Yang and Yu2013) and blastocyst development (Sills et al., Reference Sills, Collins, Brady, Walsh, Marron, Peck, Walsh and Salem2011). Notably, individual follicular fluid (FF) AMH levels predicted ART outcome far better than serum AMH. Ciepiela et al. (Reference Ciepiela, Dulęba, Kario, Chełstowski, Branecka-Woźniak and Kurzawa2019) investigated the correlation between AMH levels, gonadotropins, and androgens in individual follicles, and ART outcomes. Significantly higher FF AMH and lower FF FSH concentrations were observed when treatments resulted in live births when compared with treatments in which there was no pregnancy. In addition, serum and FF AMH levels were weakly correlated, suggesting that the ovarian follicle has autonomy over its secretory products (Ciepiela et al., Reference Ciepiela, Dulęba, Kario, Chełstowski, Branecka-Woźniak and Kurzawa2019).
AMH acts by modulating FSH signalling in granulosa cells through inhibition of FSH receptor gene transcription (FSHR; Pellatt et al., Reference Pellatt, Rice, Dilaver, Heshri, Galea, Brincat, Brown, Simpson and Mason2011), and through acute suppression of FSH-induced cyclic adenosine monophosphate accumulation (Chang et al., Reference Chang, Klausen and Leung2013). Therefore, FSH has been suggested as the protagonist of the oocyte homeostasis mechanism, while AMH would play a secondary role, inhibiting the negative effect of excess FSH (Buratini et al., Reference Buratini, Dellaqua, Dal Canto, La Marca, Carone, Mignini Renzini and Webb2022).
Previous studies have demonstrated that the degree of apoptosis of granulosa cells negatively affects oocyte developmental competence (Nakahara et al., Reference Nakahara, Saito, Saito, Ito, Ohta, Takahashi and Hiroi1997; Zeuner et al., Reference Zeuner, Müller, Reguszynski and Jewgenow2003). In line with this, atretic human (Weenen et al., Reference Weenen, Laven, Von Bergh, Cranfield, Groome, Visser, Kramer, Fauser and Themmen2004) and animal (Bézard et al., Reference Bézard, Vigier, Tran, Mauléon and Josso1987; Baarends et al., Reference Baarends, Uilenbroek, Kramer, Hoogerbrugge, van Leeuwen, Themmen and Grootegoed1995) follicles do not express AMH. In addition, Fanchin et al. (Reference Fanchin, Mendez Lozano, Frydman, Gougeon, di Clemente, Frydman and Taieb2007) suggested that a direct link exists between the ability of granulosa cells to produce AMH and the oocyte function, reflected by its competence to become an embryo with high implantation potential. Our data support previous reports on the prognostic value of AMH levels on embryo quality.
The retrospective nature and relatively small number of ICSI cycles limit our study. Also, it is unknown whether significant impacts in clinical pregnancy were not noted because slower embryos are generally not selected for transfer or because there is no association between the variables at all. Therefore, a cautious interpretation is due. The results presented here contribute to the knowledge of the relationship between serum AMH levels and embryo morphokinetic development.
In conclusion, this study highlights the association between AMH and embryo morphokinetic development. Significant positive relationships were observed between embryo development and AMH levels, and differences that could not have been noticed if the embryos were being cultured in a conventional incubator. Patients with low AMH have a slower embryo development and quality according to KIDScore assessment.
Declaration of interest
All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.









