Introduction
In the mammalian testes, the seminiferous epithelium is a complex and dynamic tissue composed of germ cells and Sertoli cells surrounded by a layer of peritubular myoid cells (Kim et al., Reference Kim, Bardwell and Zarkower2007). Spermatogenesis is delicately regulated using a precise regulatory mechanism. In this process, male germ cells differentiate from spermatogonial stem cells to haploid sperm (Kimmins et al., Reference Kimmins, Kotaja, Davidson and Sassone-Corsi2004; Hai et al., Reference Hai, Hou, Liu, Liu, Yang, Li and He2014). Normal spermatogenesis depends on Sertoli cells maintaining the cell junction and supporting germ-cell mitosis, meiosis, and differentiation due to their structural, immunological, and nutritional support, which requires proper interactions between germ cells and Sertoli cells; this process is also achieved by appropriate gene expression in Sertoli cells (Kimmins et al., Reference Kimmins, Kotaja, Davidson and Sassone-Corsi2004; Bettegowda and Wilkinson, Reference Bettegowda and Wilkinson2010; Ni et al., Reference Ni, Hao and Yang2019). All these functions, to a certain extent, are assigned to the transcription factors that control the expression of various genes in spermatogenesis.
Transcription factors are key players in gene regulatory networks by binding specific sequences of genes to activate or repress gene transcription. These factors result in temporal and tissue-specific gene expression (de Mendoza and Sebé-Pedrós, Reference de Mendoza and Sebé-Pedrós2019). One such transcription factor is the zinc finger transcription factor, which is the most prominent transcription factor family of DNA-binding transcription factors in eukaryotes and is reported to participate in cell proliferation, cell apoptosis, cell migration, and numerous other processes (Seetharam and Stuart, Reference Seetharam and Stuart2013; Cassandri et al., Reference Cassandri, Smirnov, Novelli, Pitolli, Agostini, Malewicz, Melino and Raschellà2017; Huang et al., Reference Huang, Du, Wang, Ren, Yu and Yang2022). Many known zinc finger transcription factors have crucial roles in spermatogenesis (Bettegowda and Wilkinson, Reference Bettegowda and Wilkinson2010). For example, Bnc1 mutation leads to male subfertility, which is due to defective germ-cell proliferation and progressive loss of germ cells with age (Zhang et al., Reference Zhang, Chou, Haig-Ladewig, Zeng, Cao, Gerton, Dobrinski and Tseng2012). Ovo like transcriptional repressor 1 (OVOL1) is an essential regulator for male germ cells to progress through the meiotic pachytene stage; disruption of Ovol1 in mice leads to a reduced number of late-pachytene spermatocytes (Li et al., Reference Li, Nair, Mackay, Bilanchone, Hu, Fallahi, Song, Dai, Cohen and Dai2005). Gametogenetin binding protein 2 (GGNBP2) is confirmed to play a vital role in DNA double-stranded break repair during spermatocyte meiosis, and Ggnbp2-null male mice are infertile. In addition, GGNBP2 is essential for spermiogenesis, and its deficiency causes the failure of round spermatids to develop into elongated spermatids (Liu et al., Reference Liu, He, Guo, Zhou, Li, Tseng, Cai, Lan, Zhou, Wang and Lei2017; Guo et al., Reference Guo, He, Liu, Liang, Li, Cai, Lan, Zhou, Wang and Lei2018).
RAS responsive element binding protein 1 (RREB1), a member of the zinc finger transcription factor family, is evolutionarily conserved (Tupler et al., Reference Tupler, Perini and Green2001). In mammals, Rreb1 is widely expressed in multiple tissues, including reproductive organs (the Mammalian Reproductive Genetics Database http://mrgd.org), and it has been confirmed to play an important role in various physiological and pathological processes in mammals, including colorectal cancer, pancreatic cancer, type 2 diabetes, and embryonic development (Li et al., Reference Li, Wang, Zhang, Lin, Zhang, Zhang, Zhang, Lu, Zheng and Li2018; Hui et al., Reference Hui, Ji, Xu, Wang, Ma, Zhang, Wang and Zhou2019; Deng et al., Reference Deng, Xia, Zhang, Ejaz and Liang2020; Morgani et al., Reference Morgani, Su, Nichols, Massagué and Hadjantonakis2021). Whether RREB1 plays a role in reproduction is still unknown. The Drosophila homolog of Rreb1, Hindsight (Hnt), was reported to be involved in the development and function of anterior follicle cells in the ovary by mediating cell adhesion to the correct position (Melani et al., Reference Melani, Simpson, Brugge and Montell2008), which indicates the potential role of RREB1 in cell adhesion (intercellular action) and female reproduction.
Due to its expression in reproductive organs, whether RREB1 plays a role in mammalian reproduction and whether this role is related to intercellular adhesion and action remain unknown. A study in pancreatic adenocarcinoma (PDA) cells confirmed that RREB1 interacts with SMAD family member 3 (SMAD3; Su et al., Reference Su, Morgani, David, Wang, Er, Huang, Basnet, Zou, Shu, Soni, Hendrickson, Hadjantonakis and Massagué2020), and Itman et al. (Reference Itman, Wong, Hunyadi, Ernst, Jans and Loveland2011) reported that SMAD3 is closely related to the development and function of Sertoli cells in the testis. Sertoli cells play important roles in spermatogenesis, and both abnormal maturation and function result in spermatogenesis and male fertility defects (Cheng and Mruk, Reference Cheng and Mruk2002; Mruk and Cheng, Reference Mruk and Cheng2004; Kopera et al., Reference Kopera, Bilinska, Cheng and Mruk2010; Griswold, Reference Griswold2018). Therefore, these preliminary findings suggest that RREB1 plays a potential role in male reproduction. Whether RREB1 participates in the maturation and function of Sertoli cells and further regulates spermatogenesis by influencing the interaction of Sertoli–germ cells should be further researched.
Collectively, the biological importance of RREB1 in mammalian reproduction is largely unknown. Our study used intratesticular injection of siRNA against Rreb1 for an initial study in mice, as the work done by Morgani et al. (Reference Morgani, Su, Nichols, Massagué and Hadjantonakis2021) showed that loss of Rreb1 causes embryos to die at midgestation.
Materials and methods
Animals
This study was carried out under the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Nanjing Medical University. Male ICR/JCL mice were kept under an SPF environment of 20–22°C, with a standard light/dark cycle, 60 ± 10% humidity, and food and water ad libitum.
Intratesticular injection of siRNA against Rreb1
The siRNAs against Rreb1 (Tsingke Biotechnology Co., Ltd., Shanghai, China) were diluted (20 mM) and stored at −20°C. The efficacies of the siRNAs were validated using the GC2 cell line according to the relevant instructions. The mice were anaesthetised with tribromoethanol (Easy Check, Shenzhen, China) and the testes exposed. siRNA mixed with 0.1% Fast Green FCF (Sangon Biotech Co., Ltd., Shanghai, China) was injected into the testis, and negative control siRNA (NC) was injected into the control testis (Figure 1a). After injection, the testes were taken back and the incisions sutured. The RREB1 proteins were observed to be significantly inhibited in the testis 48 h after siRNA injection (Figure 1b). The subsequent in vivo study was performed under the same conditions.

Figure 1. Knockdown strategy. (a) Schematic diagram of intratesticular injection. (b) Western blotting analysis detected the efficiencies of Rreb1 siRNA in vivo 48 h after injection (left), densitometric analysis of western blot signals (right) (n = 3, **P < 0.01).
RNA isolation, cDNA synthesis, PCR and quantitative real-time PCR analyses
According to the manufacturer’s instructions, total RNAs extracted from mice testes using TRIzol reagent (Invitrogen, California, USA) were reverse transcribed to cDNA using HiScript II Q Select RT SuperMix (Vazyme Biotech Co., Ltd, Nanjing, China). cDNAs were amplified using 2× Taq Plus Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China) with specific primers. The PCR products were separated using agarose gel electrophoresis and normalized using GAPDH. cDNAs were carried out using AceQ qPCR SYBR Green Master Mix (Vazyme Biotech Co., Ltd, Nanjing, China) for qRT-PCR on the ABI Q5 real-time PCR System (Applied Biosystems, Thermo Fisher Scientific, Massachusetts, USA). The primer sequences are listed in Table S1.
Western blotting analysis
Total protein from the testis was extracted using RIPA lysis buffer (Beyotime, Shanghai, China) adding 1% protease inhibitor cocktail (Bimake, Texas, USA). The protein samples were mixed with sample buffer (NCM Biotech, Suzhou, China) and boiled at 100°C for 5 min. The samples were electrophoresed on SDS-PAGE gels and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, California, USA), which were blocked in Tris-buffered saline (TBS) containing 5% nonfat dried milk powder for 2 h at room temperature and incubated overnight with specific primary antibody diluted in Tris-buffered saline/Tween (TBST) at 4°C. Gapdh was used as a protein loading control. The secondary antibodies were conjugated with the washed membranes. Specific proteins were visualized using a Bio-Rad gel imaging system. The primary and secondary antibodies and their applications are listed in Table S2.
Histological analysis
Testes were fixed in modified Davidson’s fluid, dehydrated and embedded in paraffin. Paraffin-embedded tissues were sectioned serially (5-μm thick). For histology, tissue samples were stained with haematoxylin and eosin (H&E) following our previously published protocols (Zheng et al., Reference Zheng, Chen, Cui, Li, Dai, Yue, Zhang, Zheng, Guo and Zhu2021). Each stage showing a distinct ordering of cell associations along the length of the seminiferous tubules was designated with Roman numerals, and approximately 200 tubules from each sample from each group were analyzed under microscopy (Ahmed and de Rooij, Reference Ahmed and de Rooij2009). The cauda epididymis sperm were collected to prepare sperm smears, fixed with 4% paraformaldehyde, and stained using H&E for morphological observation. Two hundred sperm were evaluated in each slide in a double-blinded method.
Immunofluorescence (IF) analyses
The paraffin sections were blocked with 2% bovine serum albumin, incubated with primary antibody (Anti-RREB1 antibody: Bioss bs-18856R; Anti-VIMENTIN antibody: Abcam ab8978) and normal IgG (Beyotime, Shanghai, China) for 12–16 h at 4°C, three washes with phosphate-buffered saline (PBS), incubated with FITC-labelled secondary antibodies for 2 h, and three washes with PBS. The nuclei were visualized with Hoechst H33342 (Sigma–Aldrich, St. Louis, USA). All slides were observed under a fluorescence microscope (Zeiss, Oberkochen, Germany).
Transmission electron microscopy analysis
Testes and sperms were collected from the NC and siRNA groups after siRNA injection and fixed for 12 h in 2.5% glutaraldehyde. They were fixed in 1.0% OsO4 after twice washing with phosphate buffer, dehydrated and embedded in Epon/Araldite resin. Thin sections were cut and mounted on 200-mesh grids, and stained with uranyl acetate and lead citrate.
Computer-assisted sperm analysis
Spermatozoa were collected from 6-week-old mice cauda epididymis dispersed in 200 μl of PBS. A 10-μl aliquot of the gently mixed sperm suspension was subjected to computer-assisted sperm analysis (CASA) detection, using the IVOS II™ system (Hamilton Thorne, USA).
Luciferase reporter assay
The promoter of mouse Wt1 and the coding sequence (CDS) region of mouse Rreb1 were constructed into the pGL3-Basic vector (Promega, Madison, WI, USA) or pcDNA3.1 vector. The luciferase reporter constructs were co-transfected into 293T cells (human kidney cells) with pcDNA3.1-Rreb1 or control using Hieff Trans™ (Yeasen, Shanghai, China). The Dual-Luciferase Reporter Gene Assay Kit (Yeasen, Shanghai, China) was used to measure the luciferase activities following the manufacturer’s protocol. Renilla luciferase was used as an internal control.
Statistical analysis
Differences between the two groups were performed using one-way analysis of variance (ANOVA) or Student’s t-test with a P-value < 0.05 considered statistically significant and reported as the mean plus standard error of the mean (SEM; GraphPad Prism 8.2.1, San Diego, USA).
Results
Expression and localization of Rreb1 in mouse testis
Gene expression data available from Mouse Genome Informatics (http://www.informatics.jax.org) indicated that Rreb1 was expressed in the testis. Using RT-PCR and western blotting, we confirmed that RREB1 was stably expressed in the mouse testis (Figure 2a). We further examined the expression of Rreb1 using testis mRNA obtained from different week-old mice and performed the comparisons among different weeks. The results showed that Rreb1 was highly expressed in the first 3 weeks and maintained a certain level of expression after 4 weeks (Figure 2b), which was consistent with the higher proportion of Sertoli cells in the testis during the first 3 weeks. To detect Rreb1 expression in specific testicular cells, we performed qRT-PCR using isolated testicular cells. The results showed the predominantly expression of Rreb1 in Sertoli cells, and obviously weak expression in spermatogenic cells (Figure 2c). At the same time, an immunofluorescence analysis with adult mice testis was performed. Based on the co-localization signals with a specific marker of Sertoli cells, we demonstrated that RREB1 was mainly expressed in the Sertoli cells (Figure 2d). The results suggested the potentially important role of RREB1 in spermatogenesis, especially in the development and function of Sertoli cells.

Figure 2. Expression characteristics of Rreb1 in mouse testes. (a) RT-PCR and western blot analysis of Rreb1 expression in mouse testes (1, 2: testicle samples from two mice). (b) Expression of testicular Rreb1 mRNA at the indicated time points after birth was examined using qRT-PCR (n = 3, ****P < 0.0001, **P < 0.01, *P < 0.05). (c) Expression of testicular Rreb1 mRNA in somatic cells and spermatogenic cells was examined using qRT-PCR (n = 6, **P < 0.01, *P < 0.05). ES: elongated spermatid; LC: Leydig cells; PS: pachytene spermatocytes; RS: round spermatid; SC: Sertoli cells; SSC: spermatogonial stem cells. (d) Immunofluorescence staining of testis sections from adult mice for RREB1 (yellow) and VIMENTIN (red), Sertoli cell cytoplasm co-staining using RREB1 and VIMENTIN (orange), nuclei counterstaining using Hoechst (blue) and acrosome staining using PNA-FITC (green). Each image exhibits a stage of the seminiferous epithelial cycle, and they are denoted by Roman numerals at the top of each image (n = 3, scale bars: 10 µm). NC: negative control.
RREB1 is essential for the functional maturation of Sertoli cells
To investigate the role of RREB1 in the functional maturation of Sertoli cells, we selected mice for study at 2 weeks postnatally (at this time, Sertoli cells begin to develop). We injected siRNA into one testis of the mouse, and an equal volume of NC was injected into the other. At 1 week post-siRNA injection (theoretically, Sertoli cells mature at this time), we assessed the state of the testes and Sertoli cells. The testes morphology was first observed under a light microscope, and vacuolations of seminiferous epithelia were found in the siRNA group testes, but there were no obvious abnormalities in the NC group (Figure 3a). Then, we measured the related molecules that marked the maturation of Sertoli cells in the testes of these mice, including anti-Müllerian hormone (Amh), androgen receptor (Ar), GATA binding protein 1 (Gata1), and WT1 transcription factor (Wt1). Amh levels were not different between the siRNA and NC groups, whereas Ar, Gata1, and Wt1 mRNA expression was significantly downregulated in the siRNA group testes (Figure 3b). Furthermore, we evaluated the blood–testis barrier (BTB) structure that is unique to mature Sertoli cells. Transmission electron microscopy (TEM) revealed a disrupted BTB structure with blisters and discontinuity in the siRNA group testes compared with the intact BTB in the NC group (Figure 3c). In addition, we examined the mRNA levels of two major BTB components, Gap junction protein alpha 1 (Gja1) and Tight junction component (Claudin11), and found that they were significantly reduced in the testes of the siRNA group (Figure 3d). Collectively, our data implied that the maturation of Sertoli cells was defective in the siRNA group.
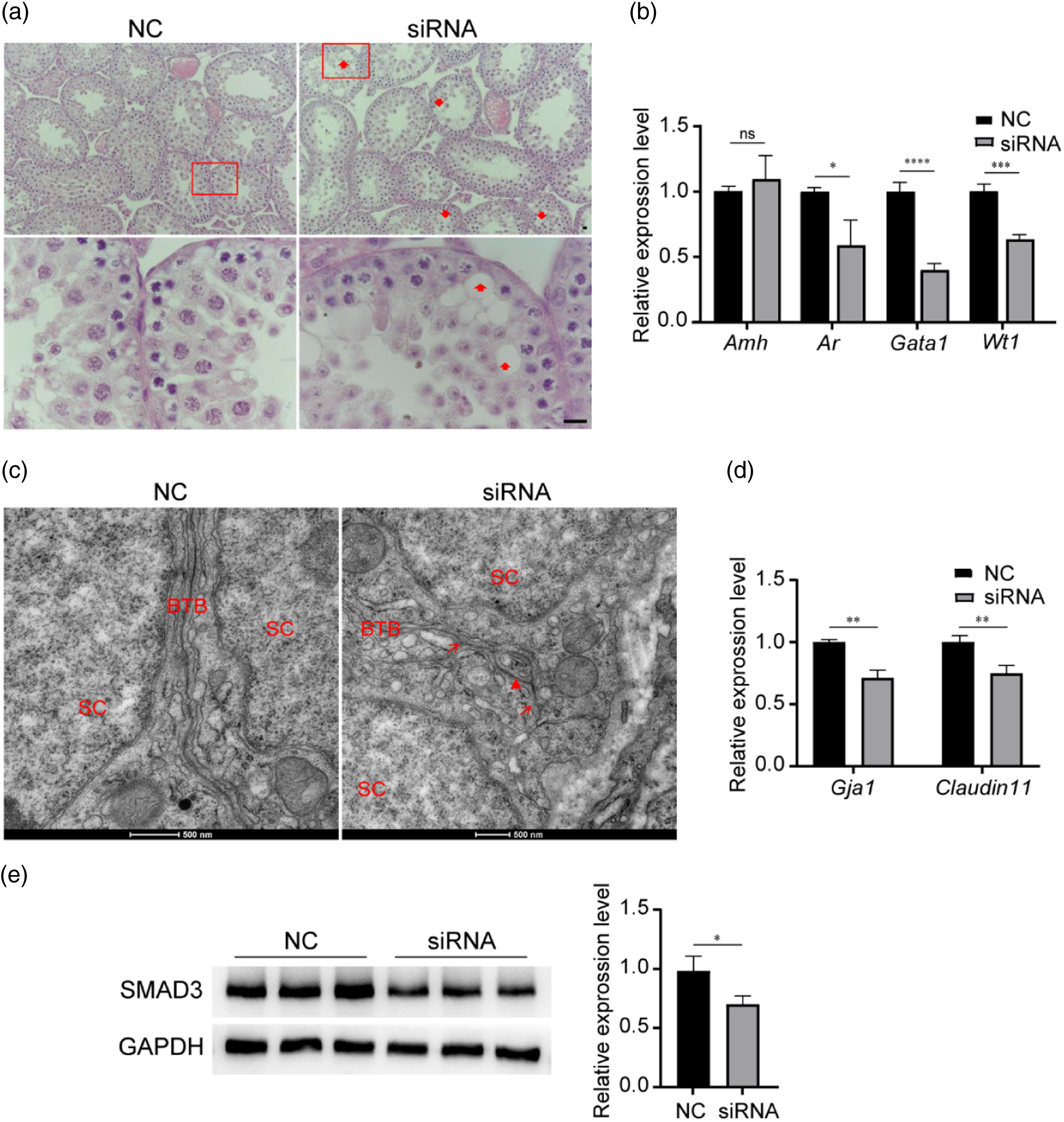
Figure 3. Low expression of RREB1 leads to impaired Sertoli cell maturity. (a) H&E staining for the observation of testicular histological structure (n = 3, red arrowheads: vacuolations of seminiferous epitheliums, scale bar: 20 µm). (b) qRT-PCR showed the levels of Sertoli cell functional maturation marker genes (n = 3, ****P < 0.0001, ***P < 0.001, *P < 0.05). (c) TEM showed the BTB structure (red triangle, and red arrows: disrupted BTB structure, scale bars: 500 nm). BTB: blood–testis barrier; SC: Sertoli cell. (d) qRT-PCR showed the levels of major BTB component genes. (n = 3, **P < 0.01). (e) Western blotting analysis detected the expression of SMAD3 (left), densitometric analysis of western blot signals (right) (n = 3, *P < 0.05).
It has been reported that a threshold level of SMAD3 is the essential factor ensuring Sertoli cell development and functional maturation (Itman et al., Reference Itman, Wong, Hunyadi, Ernst, Jans and Loveland2011; Nicholls et al., Reference Nicholls, Stanton, Chen, Olcorn, Haverfield, Qian, Walton, Gregorevic and Harrison2012). Consequently, we detected the expression of SMAD3 and found that it was significantly decreased after treating 2-week-old testes with siRNA for 48 h (Figure 3e). This phenomenon might be the cause for Sertoli cells failing to mature.
Downregulation of RREB1 causes a reduction in sperm quantity and quality
When the mice were 3 weeks old, Sertoli cells became mature, and they switched their role to support spermatogenesis. At this time, round spermatids appeared in the first wave of spermatogenesis, and it took approximately another 3 weeks to transport sperm to the epididymis cauda.
To clarify the role of RREB1 in Sertoli cell function and its further effect on spermatogenesis, we first observed the effect of RREB1 reduction on spermatogenesis. We injected Rreb1 siRNA into one testis of a 3-week-old mouse and an equal volume of NC into the other testis. After 3 weeks, epididymal sperm were collected for assessment using CASA, showing that the sperm number of the siRNA group was significantly lower than that of the NC group (Figure 4a). Moreover, the state of spermatogenic cells in the testes of these mice was evaluated, and significantly increased cell apoptosis was found in siRNA group mice using terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) analysis (Figure 4b,c), which corresponds to the reduction of epididymis sperm. The CASA results also showed that the sperm motility and progressive motility of the siRNA group were significantly lower than those of the NC group (Figure 4a).
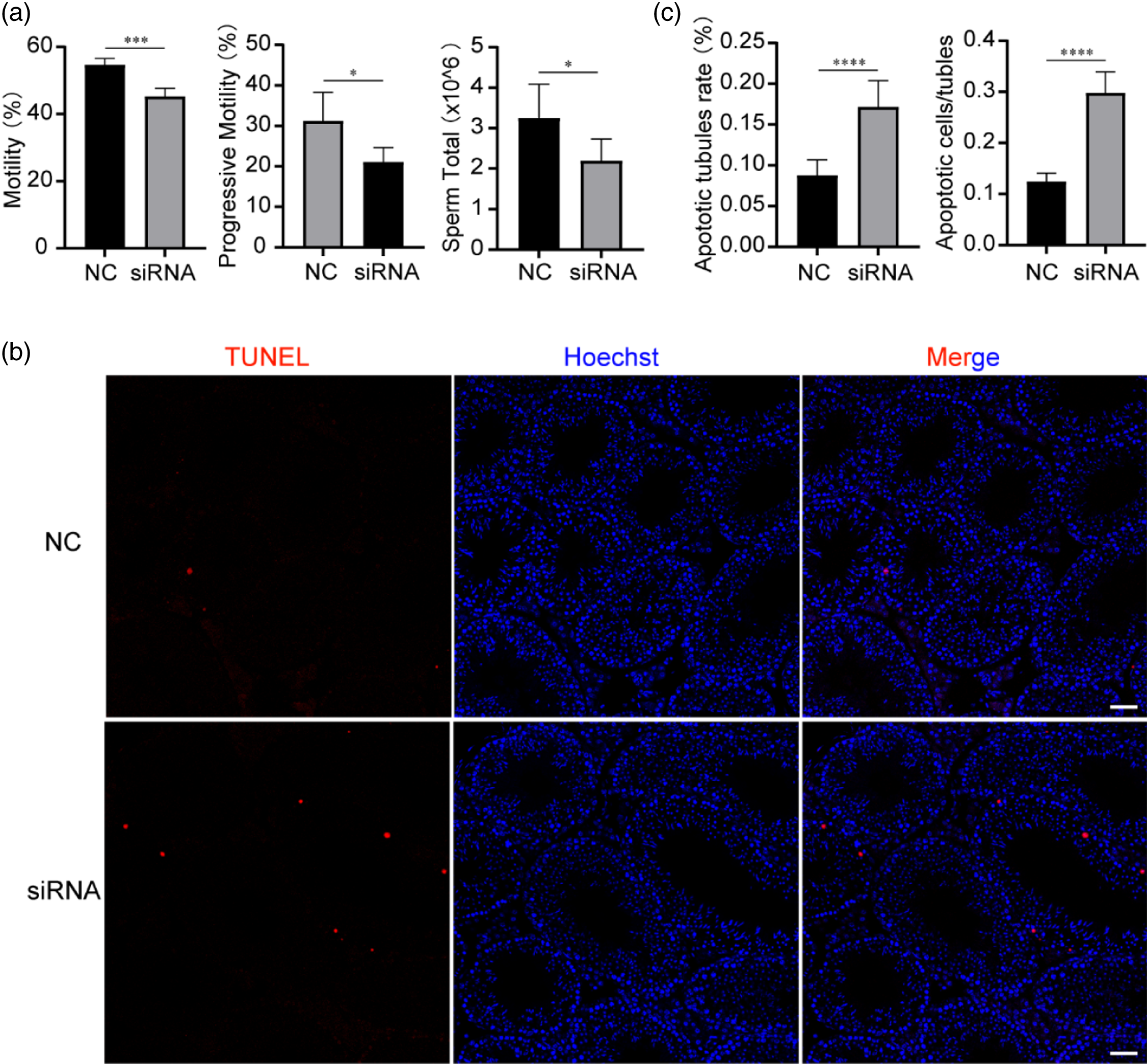
Figure 4. Low expression of RREB1 leads to increased spermatogenic cell apoptosis and decreased sperm quantity and motility. (a) Computer-assisted sperm analysis (CASA) of sperms from cauda epididymides was performed (n = 5, ***P < 0.001, *P < 0.05). (b) Terminal deoxynucleotidyl transferase nick-end labelling (TUNEL) staining (red) in testes. Nuclei are stained with Hoechst (blue) (n = 5, scale bars: 20 µm). (c) Analysis of the apoptotic tubule rate and the number of apoptotic cells per tubule (n = 5, ****P < 0.0001, ***P < 0.001).
In addition, we observed the morphology of spermatozoa from the siRNA and NC groups. Under the light microscope, compared with the normal morphology of sperm from the NC group, the sperm from the siRNA group showed a significant increase in sperm tail defects, manifested as tail folding and curling (Figure 5a). According to the statistics, the siRNA group had a higher deformity rate than the NC group (Figure 5b). We further compared the ultrastructure of the sperm from the two groups using TEM. The sperm from the NC group showed normal condensed nuclei and smooth tails. However, two or more cross-sections of the sperm flagellum in one cell membrane and abnormal nuclei with vacuoles were frequently found in the siRNA group (Figure 5c).
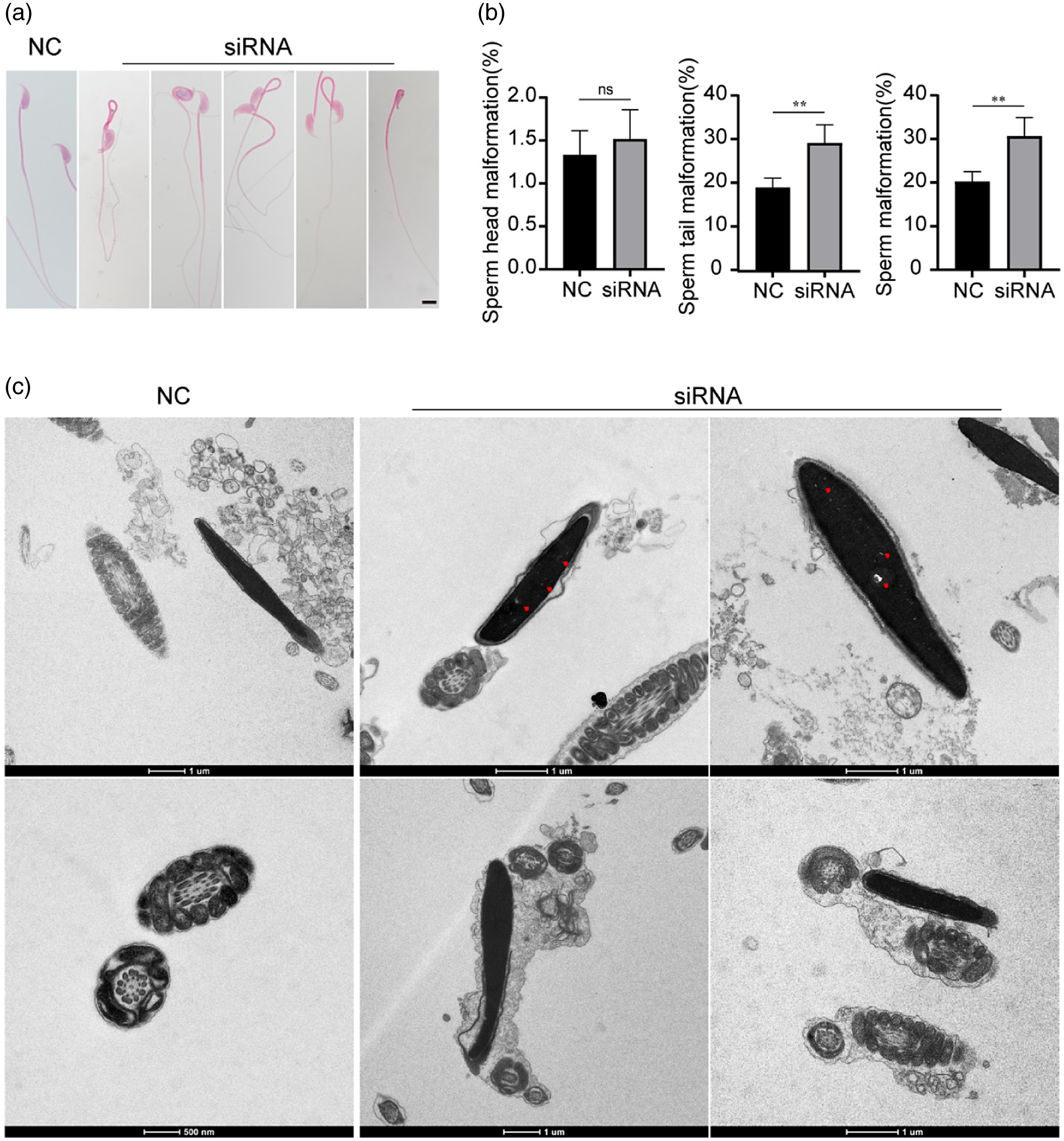
Figure 5. Low expression of RREB1 leads to impaired sperm morphology and structure. (a) Representative images of epididymal sperm under a light microscope (n = 5, scale bar: 10 µm). (b) The percentage of abnormal spermatozoa with irregular morphology (n = 5, **P < 0.01). (c) TEM images of sperm (red triangles: abnormal nuclei with vacuoles, scale bars: 1 µm, 500 nm).
Downregulation of RREB1 causes defects in Sertoli cells, which influences the Sertoli–germ-cell interaction
To determine whether abnormal spermatogenesis after RREB1 reduction is related to Sertoli cell defects, we observed the ultrastructure of Sertoli cells. The TEM results showed an aberrant BTB with the occurrence of blisters in the testes of the siRNA group, while there was no obvious abnormality in the BTB of the NC group (Figure 6a), which confirmed the defect of Sertoli cells.

Figure 6. Low expression of RREB1 leads to impaired Sertoli cell structure and function. (a) TEM showed the BTB structure (red triangle, and red arrowheads: disrupted BTB structure, scale bar: 500 nm). BTB: blood–testis barrier; SC: Sertoli cell. (b) H&E staining showed the histological structure of testes (left). Analysis of the abnormal lumen rate (right) (n = 3, **P < 0.01; red arrowheads: vacuolations of seminiferous epitheliums, scale bar: 20 µm).
Sertoli cells act as the support structure of germ cells, forming various dynamic connections with germ cells to regulate spermatogenesis (Wu et al., Reference Wu, Yan, Ge and Cheng2020). The shed germ cells might indicate the impaired interaction of the Sertoli–germ cells. We conducted a histological examination of the testes and found an obvious loss of germ cells and increased vacuoles in the seminiferous tubules of the siRNA group, whereas the NC testis was normal (Figure 6b). The results suggested that the defect of Sertoli cells and their impaired interaction with germ cells might be the basic reason for abnormal spermatogenesis after RREB1 reduction.
The potential mechanism by which RREB1 affects Sertoli cell function and participates in spermatogenesis
A previous study demonstrated that follicle-stimulating hormone receptor (Fshr) was directly targeted by RREB1(Xing and Sairam, Reference Xing and Sairam2002). FSHR is specifically expressed and plays an important role in Sertoli cells, participating in spermatogenesis. FSHR was reported to be essential for sustaining the proper shapes of sperm heads and tails (Grover et al., Reference Grover, Smith, Gregory, Cyr, Sairam and Hermo2005). In this study, we found that the expression of FSHR was significantly decreased at 48 h after the knockdown of Rreb1 in the testes of 3-week-old mice (Figure 7a), which might be the potential mechanism for the abnormal increase in sperm morphology after Rreb1 knockdown.
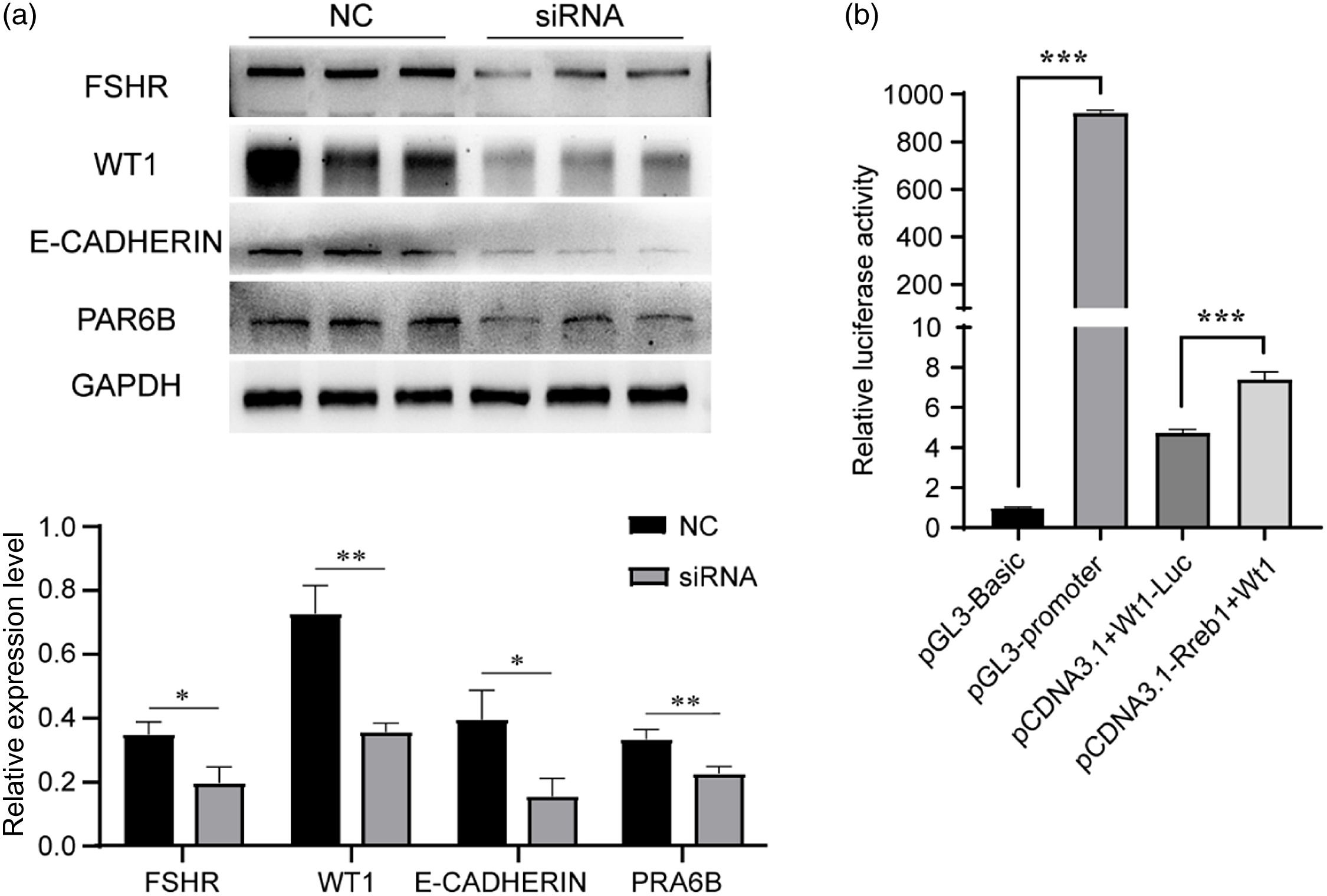
Figure 7. The potential mechanism by which RREB1 affects Sertoli cell function and participates in spermatogenesis. (a) Western blotting analysis detected the expression of FSHR, WT1, E-CADHERIN, and PRA6B (above), densitometric analysis of western blot signals (below) (n = 3, **P < 0.01, *P < 0.05). (b) Dual-luciferase activity assay (***P < 0.001).
Wt1 is another gene that is specifically expressed in Sertoli cells and might play an essential role in Sertoli cell structure and function (Wang et al., Reference Wang, Li, Ren, Jiang, Wang, Chen, Zhang, Hao, Wang, Sha, Huang, Liu, Hu, Sun, Li, Xiong, Xie, Jiang and Cai2013). It has been reported that the knockdown of Wt1 in testes caused increased germ-cell apoptosis and reduced sperm count and motility. The knockout of Wt1 in mice resulted in the downregulation of polarity-associated genes (Par6b and E-cadherin). This altered expression subsequently led to abnormal Sertoli cell polarity characteristics, disrupted the normal BTB structure, and eventually caused germ-cell death (Wang et al., Reference Wang, Li, Ren, Jiang, Wang, Chen, Zhang, Hao, Wang, Sha, Huang, Liu, Hu, Sun, Li, Xiong, Xie, Jiang and Cai2013). We found that the protein expression of WT1 and the polarity-associated proteins Par-6 family cell polarity regulator beta (PAR6B) and Epithelial cadherin 1 (E-CADHERIN) was reduced in the siRNA group testicular tissue (Figure 7a), which might be the potential mechanism for the increased germ-cell death and reduced sperm number and motility after Rreb1 knockdown.
To determine whether Wt1 was directly targeted by RREB1, we transiently transfected 293T cells with wild-type mouse Rreb1 (pcDNA3.1-Rreb1) overexpression plasmid or with either empty vector (pcDNA3.1) together with a Wt1-luciferase (Wt1-Luc) promoter reporter construct. A dual-luciferase reporter assay demonstrated that Rreb1 treatment increased the luciferase activity of Wt1-Luc. The data indicated that Rreb1 activated Wt1 expression by directly recognizing the promoter of Wt1 mRNA (Figure 7b).
Discussion
During the early postnatal period, Sertoli cells undergo proliferation and functional maturation and, after that, the role of Sertoli cells switches to support spermatogenesis (Sharpe et al., Reference Sharpe, McKinnell, Kivlin and Fisher2003; Griswold, Reference Griswold2018). Sertoli cells are the only somatic cells in direct contact with germ cells and play an important role in the normal occurrence of spermatogenesis, which also determines the sperm number (Orth et al., Reference Orth, Gunsalus and Lamperti1988). In the present study, we showed that RREB1 is critical to Sertoli cell maturation and function and further influences spermatogenesis. We acquired Rreb1 knockdown mice in age-specific mice by injecting siRNA against Rreb1 to study the function of RREB1 in male reproduction. The introduction of siRNA into Sertoli cells decreases the target mRNA, and new mRNA transcription correspondingly stops. Therefore, the levels of protein expressed via the target gene can be repressed for a relatively long period (Aigner, Reference Aigner2006).
It has been reported that a threshold level of SMAD3 is the essential factor ensuring Sertoli cell development and functional maturation (Itman et al., Reference Itman, Wong, Hunyadi, Ernst, Jans and Loveland2011; Nicholls et al., Reference Nicholls, Stanton, Chen, Olcorn, Haverfield, Qian, Walton, Gregorevic and Harrison2012). RREB1 is a key partner of SMAD3; our data showed that at the initiation of Sertoli cell development (2 weeks of age), the knockdown of Rreb1 led to a decrease in SMAD3 expression, which might have led to defects in the functional development of Sertoli cells. The abnormal Sertoli cell development after RREB1 knockdown was further confirmed by the changes in testicular morphology and related molecules. We found that at the stage of Sertoli cell functional maturation (3 weeks of age), the testicular mRNA levels of Sertoli cell functional maturity markers such as Ar, Gata1, and Wt1 were significantly downregulated in siRNA group testes compared with those of the NC group. In addition, BTB, as a structural marker of mature Sertoli cells, was found to be defective in the siRNA group. Microscopic observation showed discontinuity of the BTB; at the same time, the expression levels of the BTB component molecules Gja1 and Claudin11 decreased significantly. The above results suggested impaired development and functional maturation of Sertoli cells in Rreb1 knockdown testes due to SMAD3 destabilization.
Mature Sertoli cells are polarized epithelial cells that provide nutrition and structural support for germ cells and create an immunological barrier called the BTB. Loss of epithelial characteristics of Sertoli cells would result in abnormal spermatogenesis in turn (Mruk and Cheng, Reference Mruk and Cheng2004). We interfered with the expression of Rreb1 in 3-week-old testes when Sertoli cells had completed their development. Interestingly, at 3 weeks post-siRNA injections, we observed abnormalities in spermatogenesis. On the one hand, the number of epididymal sperm was significantly decreased, which might be associated with the significantly increased germ-cell apoptosis in the testes. On the other hand, the quality of sperm was also significantly decreased, including reduced motility and increased sperm malformation. Moreover, the testes of these mice showed phenotypes of Sertoli cell damage, such as spermatogenic epithelial vacuoles and abnormal BTB structure. Therefore, we believe that spermatogenesis disorders are due to defects in the structure and function of Sertoli cells and that RREB1 plays an essential role in Sertoli cell function.
Fshr is expressed exclusively in Sertoli cells in males, and Fshr signalling is considered essential for the maintenance of normal spermatogenesis (Dierich et al. Reference Dierich, Sairam, Monaco, Fimia, Gansmuller, LeMeur and Sassone-Corsi1998; Wang et al., Reference Wang, Li, Yang and Tan2022). In some earlier studies on Fshr-null mice spermatogenesis, abnormal shapes of heads and nuclei, sperm tails with two axonemes and associated structures surrounded by a common cytoplasm were noted (Xing and Sairam, Reference Xing and Sairam2002), which is similar to the phenotype of our Rreb1 knockdown mice. Furthermore, a previous study demonstrated that RREB1 could activate Fshr promoter activity (Wu et al., Reference Wu, Yan, Ge and Cheng2020). Therefore, Fshr is most likely to be a transcriptional regulation target of RREB1 participating in spermatogenesis. In this study, we demonstrated decreased expression of FSHR after the knockdown of RREB1, indicating that RREB1 participates in shaping sperm morphology by directly regulating the expression of FSHR.
A previous study demonstrated that Wt1 knockdown mice showed increased germ-cell apoptosis and reduced sperm count and motility (Rao et al., Reference Rao, Pham, Imam, MacLean, Murali, Furuta, Sinha-Hikim and Wilkinson2006), and we found consistent phenotypes in Rreb1 knockdown mice. Whether Wt1 is another regulatory target of RREB1 participating in spermatogenesis is unclear. Our results demonstrated that RREB1 could regulate Wt1 promoter activity. In addition, the knockdown of Rreb1 downregulated the expression of WT1 and its downstream target genes, Pcar6b and E-cadherin. Xing et al. reported that PAR6B and E-CADHERIN are polarity-associated proteins and important for maintaining the structural integrity of BTB restructuring. Therefore, WT1 indirectly regulates the polarity of Sertoli cells through polarity-associated proteins. Wt1-deficient mice show abnormal Sertoli cell polarity, which affects epithelial characteristics and in turn results in germ-cell death (Xing and Sairam, Reference Xing and Sairam2002). Collectively, we believe that RREB1 participates in spermatogenesis by affecting the transcription of Wt1 in Sertoli cells and that Rreb1 expression in Sertoli cells is essential to the survival of germ cells and spermatozoa motility.
In conclusion, our study demonstrates that RREB1 is essential for the development and function of Sertoli cells and is required for normal spermatogenesis. RREB1 regulates Sertoli cell functional maturation by regulating the expression of SMAD3 and plays a role in Sertoli cell function by activating the transcription of Fshr and Wt1. Rreb1 could serve as a candidate gene for the aetiology of male sterility and provide potential molecular targets for diagnosis.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0967199423000655
Acknowledgements
HZ have designed the study. ZW, XC, TY, LY and LSZ performed the experiments. HZ, ZW, XC, TY, LY, LSZ and MMZ analyzed the data. ZW and HZ wrote the original draft, produced figures and edited the manuscript. HZ critically revised the manuscript and supervised the study. All authors read and approved the final manuscript.
Financial support
This work was supported by grants from the National Natural Science Foundation of China (grant numbers: 82071702, 82271636, and 31871164).
Competing interests
None.
Ethical standard
This study was carried out in the guidelines of the IACUC of Nanjing Medical University.










