Introduction
Neonatal mortality in dogs is an important problem in breeding kennels, with 7–21% of puppies dying between birth and the three first weeks of life (defined as the neonatal period of the dog) (Indrebø et al., Reference Indrebø, Trangerud and Moe2007; Mila et al., Reference Mila, Grellet, Feugier and Chastant-Maillard2015; Chastant-Maillard et al., Reference Chastant-Maillard, Guillemot, Feugier, Mariani, Grellet and Mila2017). Most of the deaths occur during the first week after birth: between 60 and 80% of total pre-weaning mortality (Indrebø et al., Reference Indrebø, Trangerud and Moe2007; Mila et al., Reference Mila, Grellet, Delebarre, Mariani, Feugier and Chastant-Maillard2017).
Clinical signs in the sick neonate are nonspecific or even nonexistent 24 h before death in 60% of cases, which makes the diagnostic before death extremely difficult (Piegari et al., Reference Piegari, Cardillo, Alfano, Vangone, Iovane and Fusco2020). Among the clinical signs, hypoxia, hypothermia, hypoglycemia, and dehydration are most often observed. All of the abovementioned syndromes predispose puppies to bacterial infections, sepsis, and as a consequence, death (Münnich and Küchenmeister, Reference Münnich and Küchenmeister2014).
Due to a very rapid deterioration of the animal and nonspecific clinical signs in most of the cases, almost the entire diagnostic relies on the postmortem examination. This step is crucial to establish an adapted treatment and try to save the rest of the litter.
The objective of these guidelines, intended for veterinary practitioners, is to present different steps of postmortem examination of the newborn dog, in order to establish a definitive diagnosis.
Stage 1: body storage
It is of paramount importance to respect specific storage conditions of the body, to be able to interpret the lesions observed during necropsy or after additional analysis (e.g., histopathology and bacteriology). Ideally, the autopsy should be performed immediately after the animal's death to minimize postmortem tissue damage. As most of the time this is not possible, it is necessary to slow down the phenomena of autolysis and putrefaction of the body by placing it in refrigeration at +4°C, ideally in a lying position (lateral decubitus), with the limbs apart and the mouth open. Placing the body in a plastic bag should be avoided as it delays cooling. A diagnostic necropsy can be done only on a rapidly refrigerated body stored for a maximum of 2–3 days (Necropsy Guide for Dogs, Cats, and Small Mammals | Wiley, n.d.). However, if any bacteriological analysis is to be done, which is often indicated in dying newborn dogs, sampling should be performed by 12–15 h following death (Necropsy Guide for Dogs, Cats, and Small Mammals | Wiley, n.d.). Early sampling will avoid bacterial translocation from the intestines to the internal organs, and so avoid tissue contamination by the digestive flora. Furthermore, it is essential not to freeze the body, as the induced changes, in particular the diffusion of hemoglobin released by lysed blood cells, largely interferes with the interpretations of macroscopic and microscopic observations.
Stage 2: clinical history
Animal history
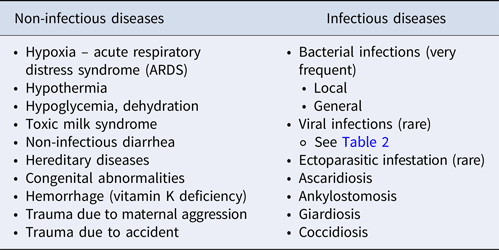
The information collected from the breeder must cover the risk factors for congenital anomalies (common in newborn puppies), the medical history of the dam or other dams in the kennel, including the course of gestation, parturition and lactation, as these may be important in determining the cause of death of a newborn puppy (Table 1) (Lamm and Njaa, Reference Lamm and Njaa2012).
Table 1. Potential causes of death in the newborn dog (adapted from Münnich and Küchenmeister, Reference Münnich and Küchenmeister2014)
Breed
The breed of the puppy is important to consider in the postmortem examination, due to racial predisposition to certain birth defects: approximately 85% of all malformations are seen in purebred puppies (Nobre Pacifico Pereira et al., Reference Nobre Pacifico Pereira, Cruz dos Santos Correia, Ritir Oliveira, Bernardo, Nagib Jorge, Mezzena Gobato, Ferreira de Souza, Rocha, Chiacchio and Gomes Lourenço2019). According to Nobre Pacifico Pereira et al. (Reference Nobre Pacifico Pereira, Cruz dos Santos Correia, Ritir Oliveira, Bernardo, Nagib Jorge, Mezzena Gobato, Ferreira de Souza, Rocha, Chiacchio and Gomes Lourenço2019), and the English Bulldog breed is most predisposed to congenital malformations, with a four times higher overall risk of cleft palate, water puppy syndrome, or atresia ani, compared to other breeds. An exhaustive list of different possible congenital diseases per breed is available in Thomas et al. (Reference Thomas, Gough and O'Neill2018); however, the prevalence for most of these diseases remains unknown.
Breeding facility
Unfavorable environmental conditions, such as insufficient hygiene in the maternity area, low-ambient temperature in the nest, or even stressful conditions for the mother, can justify some hypotheses regarding cause of death, such as bacterial infections, canine herpesvirus (CHV) or abnormal maternal behavior. Vaccination history may also be useful in interpreting lesions, with a less likely risk of viral disease in puppies born to routinely vaccinated mothers.
Even if parasitosis in the neonatal period remains rarely described in the literature, it is important to evaluate the mother's deworming protocols. Lethal toxocariasis cases have been observed in puppies as early as 3–6 days after birth in kennels with marginal deworming protocols during gestation and lactation, both in dams and their offspring: larval stages of Toxocara canis accompanied by inflammatory cells were presented in lungs, liver and small intestines (Buckle et al., Reference Buckle, Hardcastle, Scott, Craig, French, Gedye and Collett2019).
History of gestation, whelping and lactation
The medical history of the bitch, but also of other bitches from the same kennel, can be important in interpreting the results obtained.
Some drugs provided during gestation (e.g. progesterone, corticosteroids, doxycycline, etc.; Meyers-Wallen, Reference Meyers-Wallen2012; Kaplan et al., Reference Kaplan, Gunther-Harrington, Sutton and Stern2018) can be teratogenic (i.e. cause congenital abnormalities) or toxic for puppies if provided to the dam during lactation (explaining growth retardation, diarrhea, neurological signs, etc.). Abortion or cases of fetal mummification in other bitches can indicate an infectious context (brucellosis, hypervirosis, neosporosis). Dystocia observed in the bitch may be the underlying cause of respiratory distress syndrome in the newborn. Finally, abnormal maternal behavior can lead to trauma (including crushes) or abandonment. In cases of absence of colostrum intake, the risk of bacterial infection is increased due to the deficit of passive immune transfer (Chastant and Mila, Reference Chastant and Mila2019). Finally, the pathologies of the mother during the postpartum period, in particular mastitis, can cause toxic milk syndrome, which is lethal for the puppy.
Clinical signs
Although clinical signs in newborn puppies are often nonspecific and the time between onset of diseases and death is often short (Münnich and Küchenmeister, Reference Münnich and Küchenmeister2014; Guerard, Reference Guerard2019; Piegari et al., Reference Piegari, Cardillo, Alfano, Vangone, Iovane and Fusco2020), the owner should be interviewed to identify specific circumstances of death. For example, if several dead puppies coming from one litter are of low birth weight, this suggests a problem during gestation (alimentary, contagious, etc.); a retarded growth of the dead puppy compared to the siblings suggests a problem since birth (hypoxia, congenital abnormalities, etc.), while sudden weight loss is often associated with an acute problem (e.g. infection or trauma). Lack of suckling reflex, weakness at birth, or hypothermia are often seen in puppies who have suffered from perinatal asphyxia. Crying after meals can suggest gastrointestinal problems. In contrast, diarrhea in the newborn is not specifically linked to a digestive disorder (Guerard, Reference Guerard2019). Finally, in cases of neonatal mortality, the clinical examination of the mother is an essential part of the clinical investigation and in particular the examination of the reproductive system (searching for mastitis, retention of the placenta, metritis, etc.).
Stage 3: necropsy
The necropsy is an examination which aims to determine the causes of death, when possible, by showing gross lesions of tissues and organs. Moreover, postmortem samples allow additional analyses such as histopathological, bacteriological and molecular (e.g., polymerase chain reaction [PCR]) diagnostic examinations of internal organs. Even if the necropsy on its own, allows us to determine the cause of death in the newborn puppy in only 22% of cases (Guerard, Reference Guerard2019), it is a first essential step in postmortem diagnostics. In addition, because the necropsy can only be performed once, it is advisable to take as many samples as possible from the cadaver and to store them. Depending on the results of the first examination, this approach would allow sequential complementary analyses.
Figure 1 describes the different stages of a necropsy, with observations of the organs and sampling for additional analyzes to minimize the risk of contamination. This procedure can be adapted according to the practitioner's suspicions: the nasal swab can be performed in case of suspicion of respiratory syndrome, while a rectal swab should be taken in case of digestive syndrome. Sampling for bacterial culture should be performed only on a fresh cadaver stored at +4 °C for a maximum of 12 h after death.
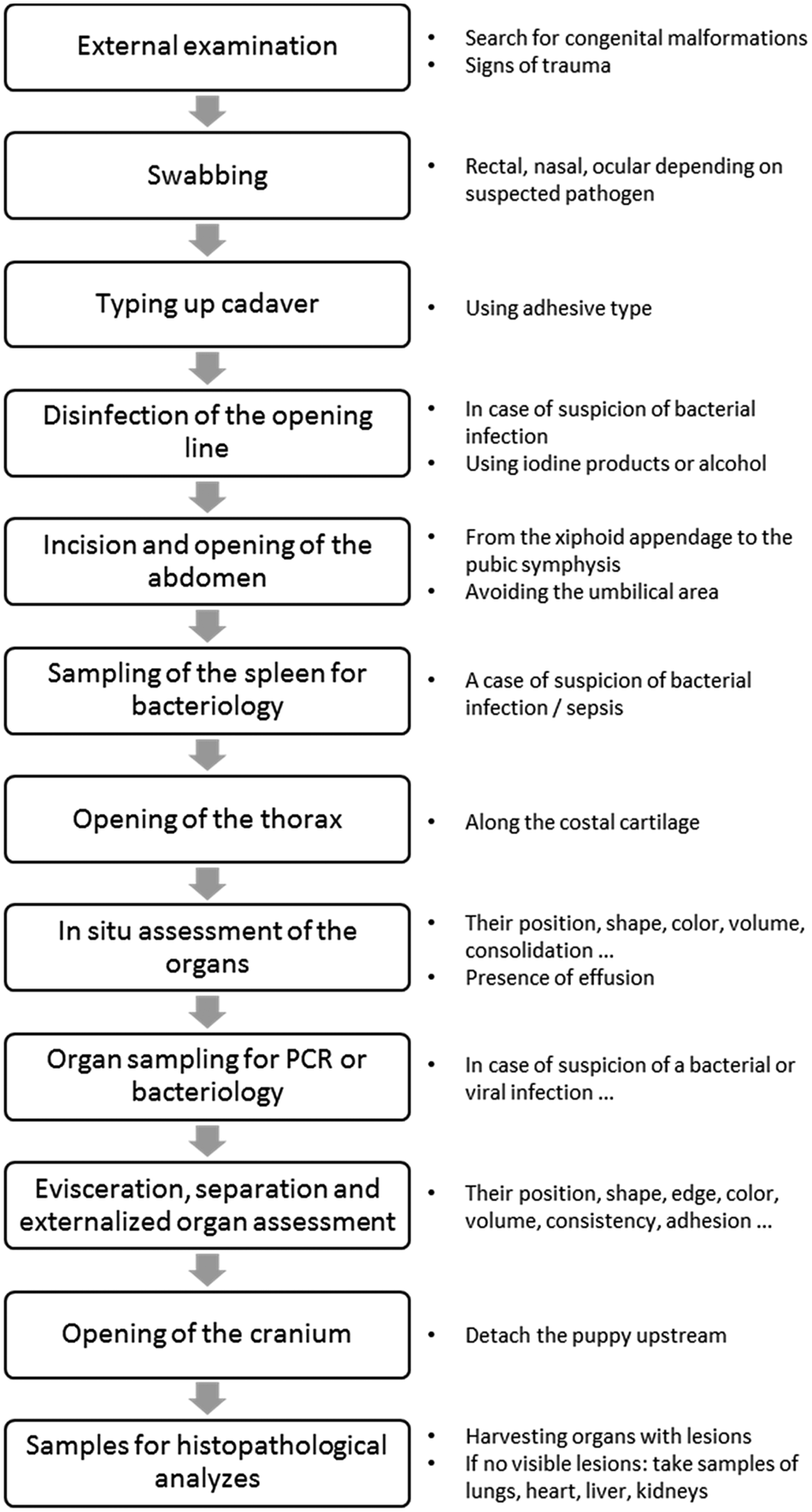
Fig. 1. Steps to follow during the necropsy of the newborn puppy.
Preparation for necropsy
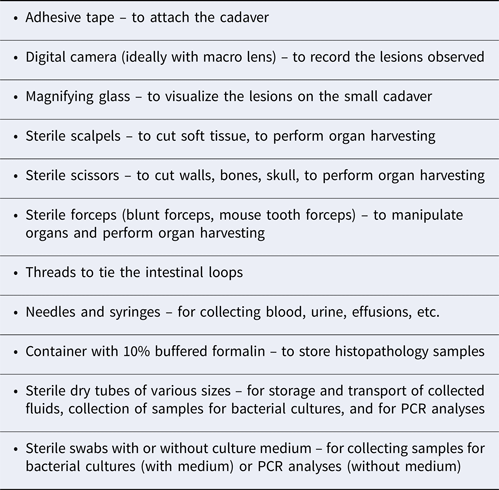
To perform a necropsy as quickly as possible, sterile equipment must be available at all times (Table 2) (Lamm and Njaa, Reference Lamm and Njaa2012). If a bacteriological analysis is to be done, some extra sterile material would be necessary.
Table 2. Equipment necessary to perform a necropsy of a newborn puppy
External examination and swabbing
Before tying up the cadaver, it is essential to weigh the puppy to assess its weight in relation to the siblings. A complete external examination will be carried out with particular attention for any sign of trauma, major anomalies, affliction of the umbilical cord (opening of the abdominal cavity, omphalitis), eye infection (neonatal ophthalmia) or the appearance of the coat (poorly developed in case of prematurity) (Blunden, Reference Blunden1988).
Some pathogens, viruses in particular, require molecular methods of detection, such as PCR. Although it is often too early to be able to target a particular infectious agent at the time of the external examination, taking a sample at this stage and storing it for subsequent analyses is strongly recommended. Indeed, the swabbing can then be carried out under good conditions with a minimal risk of contamination. A bacterial culture should be considered in cases with signs of eye infection, respiratory infection or diarrhea.
In situ assessment of the internal organs
The opening of the thoracic and abdominal cavities allows the in situ assessment of the internal organs. It allows the detection of anomalies in the relative position, shape, color or volume of organs, presence of adhesions, masses or effusions, or any other visible modifications. It must be noticed that some organs like the liver and thymus are larger in a newborn puppy.
Tissue sampling
Bacteriology
If sampling for bacterial culture is to be performed, the thorax and abdomen surface should ideally be treated with iodine products before opening both cavities. Bacterial infections are the most common cause of mortality observed during the neonatal period, responsible for 40–65% of all neonatal deaths (Indrebø et al., Reference Indrebø, Trangerud and Moe2007; Meloni et al., Reference Meloni, Martino, Grieco, Pisu, Banco, Rota and Veronesi2014) (Table 1). It is therefore reasonable to collect material for bacteriological analyses systematically in newborn puppies, especially in the absence of symptoms suggesting other pathologies. Bacterial infection is frequently followed by sepsis, which is considered a major immediate cause of death in puppies during the first 3 weeks after birth (Münnich and Küchenmeister, Reference Münnich and Küchenmeister2014). Because antemortem blood cultures are hardly ever performed in puppies suspected of having sepsis, bacteriology of the spleen is advisable in these puppies. In fact, the spleen is considered an equivalent of vascular or cardiac blood samples (Roberts, Reference Roberts1998), the latter being very difficult to obtain from small cadavers such as newborn puppies. Apart from the spleen, the organs most often harvested in puppies for bacteriology are the lung, liver, and kidney, due to their resistance to autolysis (Meloni et al., Reference Meloni, Martino, Grieco, Pisu, Banco, Rota and Veronesi2014). The choice of tissue to be taken can be made according to the lesions observed at necropsy or antemortem, but the latter only in cases with significant clinical signs. If several samples are to be taken, the organs should be removed in a predetermined order to avoid contamination: the lungs, heart, thymus, liver, spleen, kidneys, and intestines. Swabbing on the surface of organs in situ or aspiration via a fine needle, once the cavities have been opened, are methods considered to be the ‘gold standard’ in human pathology (Tsokos, Reference Tsokos2007). Once organs or organ fragments are removed, they are generally placed in sterile tubes without any growth medium.
PCR
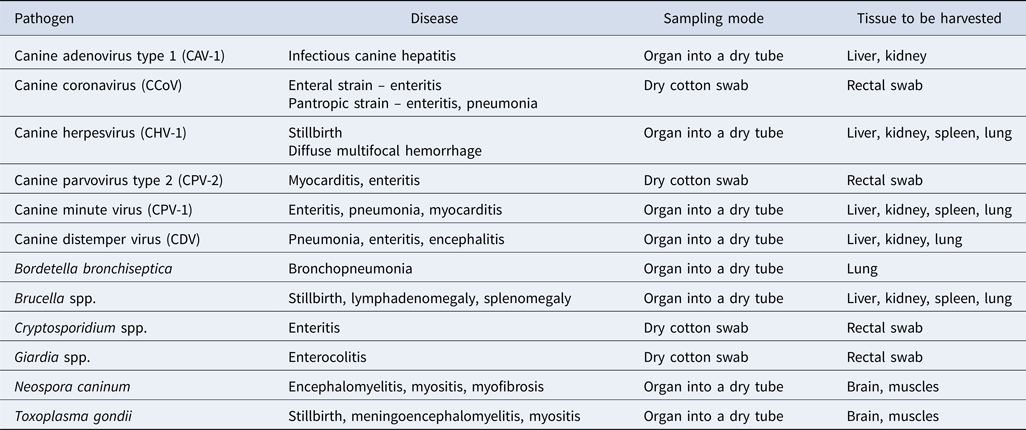
The decision of sampling for PCR analysis is made by the veterinary practitioner, according to the hypothesis established after the client interview as well as the lesions observed during necropsy. Depending on the suspected pathogens, different tissues must be sampled, which are often indicated by the laboratory performing the analyses (Table 3).
Table 3. Choice of sample for identification of pathogens by PCR (Infectious Diseases of the Dog and Cat – 4th Edition, n.d.)
Assessment of the internal organs after evisceration
Each organ is carefully examined after removal, taking into account the volume, shape, consistency and color of the entire organ or any present abnormality. The presence of various liquids, foreign bodies, or parasites should be checked in the thoracic and abdominal cavities and in the digestive contents. While the small size of the cadaver makes the necropsy of the puppy quick to perform, it also limits this examination compared to adult dogs. For example, the identification of cardiac abnormalities is difficult to carry out in puppies. To investigate small organs, a magnifying glass or binoculars may be useful. A pulmonary flotation test (hydrostatic pulmonary test) should be considered in puppies that have died within hours of birth, to determine if the puppy has breathed (stillbirth or prematurity in the event of a positive result – the fetal lungs sink to the bottom of the jar because not filled with air). When performing an necropsy, lesions should not be confused with non-lesional postmortem changes in the cadaver (Table 4).
Table 4. List of postmortem artifacts

Sampling for histopathology
Classically, all organs with macroscopic lesions should be collected for histopathological analysis. For the sample to be representative, it must be taken at the junction between the lesion and macroscopically healthy tissue. However, the macroscopic lesions in newborn puppies are often non-conclusive, making the choice of organ sampling for histopathological exam difficult. Moreover, postmortem tissue alterations are frequently marked in certain organs in canine newborns maintained at a high temperature (e.g. temperature in the nest at around 28–30 °C). Thus, autolysis appears shortly after death, making the interpretation of lesions impossible in 25% of brains collected from newborn puppies, and in 65% of duodenum samples (Guerard, Reference Guerard2019). These two organs will therefore only be chosen for histological analysis in the cadavers autopsied a maximum of few hours after death. In the absence of visible lesions, the minimum combination of organs most informative and most resistant to autolysis involves the liver, heart, lung and kidney (Guerard, Reference Guerard2019). The ideal size of the samples is 8 × 8 mm, to ensure good fixation of the tissues. However, for some organs (e.g. the digestive tract), samples 2 cm long by 0.5 cm thick are also acceptable (Necropsy Guide for Dogs, Cats, and Small Mammals | Wiley, n.d.). Several organs can be mixed in one formalin container (most frequently used as fixative), always respecting a 1:10 ratio of volume between the fixative and the organs.
Stool sample for coproscopy
Although the presence of intestinal parasites alone is rarely a cause of death in newborn puppies, they can promote the development of other pathogens and thus lead to death. Coproscopy is therefore recommended, mainly in the event of gastrointestinal lesions. Intestinal contents are simply expelled by finger pressure along the terminal portion of the digestive tract directly into an airtight container. The amount needed depends on the coproscopy technique, but it should be at least 2–5 g, or even greater in the case of watery stools due to dilution of parasites in such contents.
Conclusion of necropsy
Following the necropsy, the most important observations are grouped together in a report prior to conclusion. Examples of interpretation of the different significant macroscopic lesions in newborn puppies are given in Table 5 and Figs. 2–4.
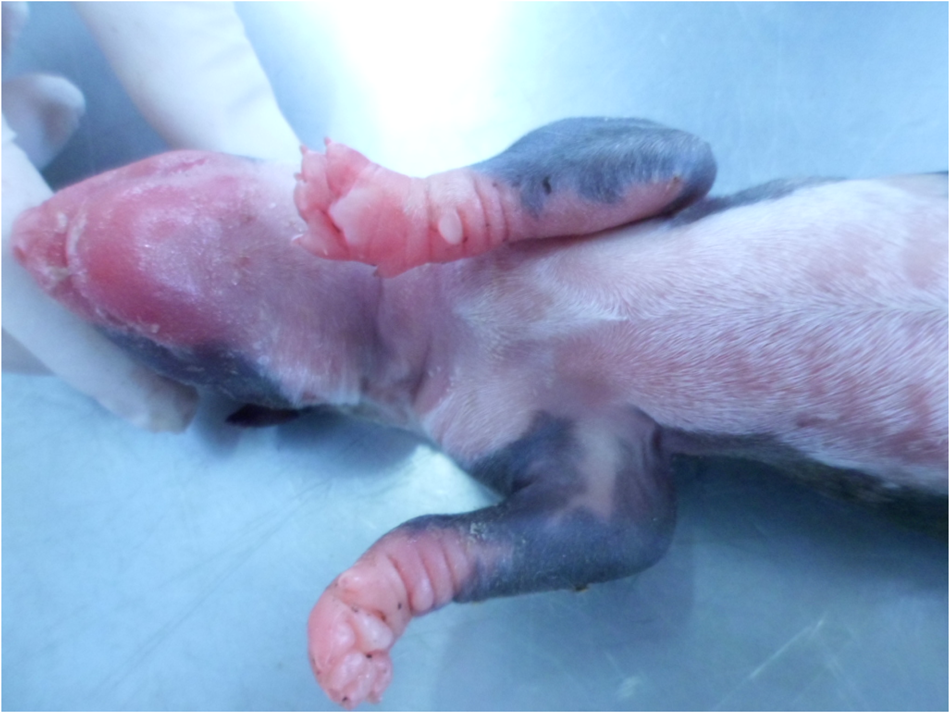
Fig. 2. Prematurity, external examination, newborn dog. Poorly developed coat of the preterm newborn.
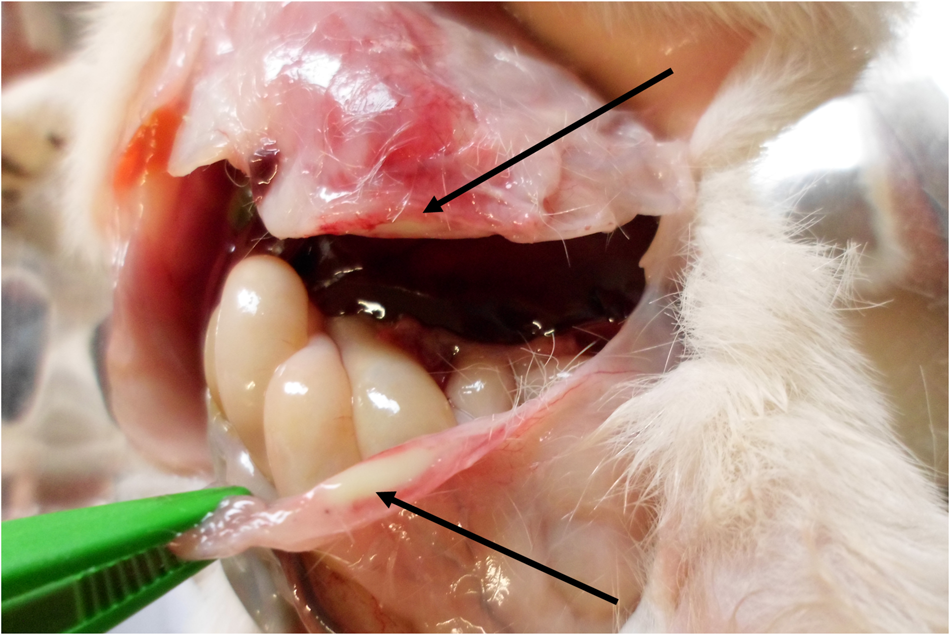
Fig. 3. Omphalitis, incision of the umbilical area, newborn dog. Purulent content and signs of inflammation in the umbilical area (arrows).
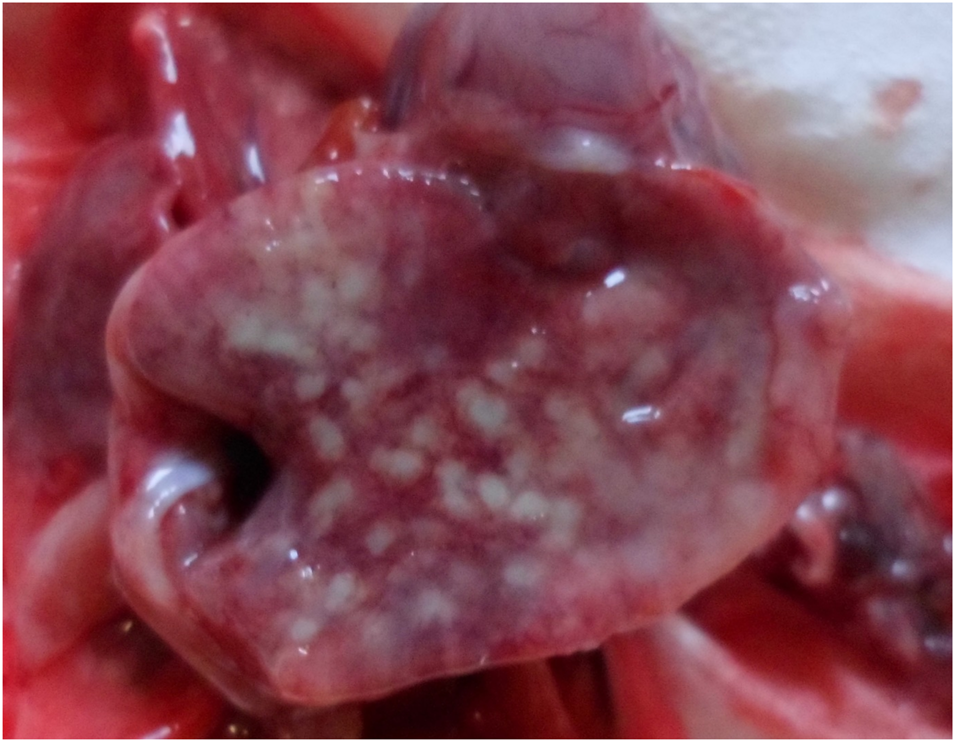
Fig. 4. Bronchopneumonia, lung, newborn dog. Multiple yellowish foci present on the surface of the right caudal pulmonary lobe.
Table 5. Common postmortem macroscopic lesions of importance for interpreting the cause of death in newborn puppies (non-exhaustive list) (Blunden, Reference Blunden1988)
a Canine adenovirus type 1.
b Canine herpesvirus.
Stage 4: storage and further sample analyses
Very often, depending on the owners' wishes and financial capacities, not all samples obtained during necropsies are sent immediately for analysis. In this section, storage conditions of different samples and sample analysis strategies will be presented.
Histopathology
Once collection and fixation have been carried out, the labeled containers should ideally be sent, along with information about the animals and suspected conditions, to a veterinary histopathological laboratory. In general, the time to obtain results is over a week; however, some laboratories currently offer results within 72 h. If immediate analysis is not possible, the formalin container can be stored at room temperature, protected from sunlight, for up to 7 days after collection.
Bacteriology
Ideally, the sample for isolation of bacteria is sent immediately, at room temperature, to the microbiological laboratory. If shipment is not possible within 2 h of collection, it is advisable to store it at +4 °C until shipment on ice packs. For most bacteria, culturing is recommended within 24 h after collection (Baron et al., Reference Baron, Miller, Weinstein, Richter, Gilligan, Thomson, Bourbeau, Carroll, Kehl, Dunne, Robinson-Dunn, Schwartzman, Chapin, Snyder, Forbes, Patel, Rosenblatt and Pritt2013). If the shipment is not possible within this period, it is possible to freeze the samples for a short time; overall, freezing at −20 °C for a period of less than 1 week does not significantly influence the results of the bacterial culture. Only certain Gram-negative bacteria such as Escherichia coli, Salmonella spp., and Pseudomonas spp., are less resistant to freezing, thus such results should be considered with caution (Alrabadi, Reference Alrabadi2015). The average time for obtaining bacteriology results is 1–2 working days.
PCR
An advantage of pathogen identification via PCR is that this technique can be performed on fresh or frozen samples. Samples can be delivered to the laboratory at the room temperature up to 12 h after collection, after which, storage at nucleic acid degradation occurs. For short-term shipment (between 12 and 48 h after sampling), samples should be shipped refrigerated. If long-term shipment (several days) is scheduled, or if samples were frozen immediately after collection, then they should be shipped frozen (Infectious Diseases of the Dog and Cat – 4th Edition, n.d.). In general, the results are obtained rapidly, between 1 and 2 working days after receipt of the samples.
Coproscopy
If the analysis of the feces is not done immediately, the sample can be stored for up to 1 week at +4 °C, protected from light. Freezing must be avoided. In suspected cases of certain fragile parasites such as Giardia spp., it is advisable to do the examination as soon as possible after collection or by another more sensitive method such as immunoassay or PCR.
Stage 5: interpretation of results of further analyses
Histopathology
Observation and interpretation of histopathological findings must be performed by certified veterinary pathologists. This is even of greater importance in the newborn puppy, as the lesions are less remarkable than in the adult dog (Figs. 5–7). The undeveloped immune systems of newborn puppies could explain less severe inflammatory reactions to infection and therefore less pronounced lesions via microscopy. In addition, the interpretation of the results may be confounded by the presence of different features of hematopoiesis, fetal structures, or foci of immature tissue, often visible during the first weeks after birth (Guerard, Reference Guerard2019).
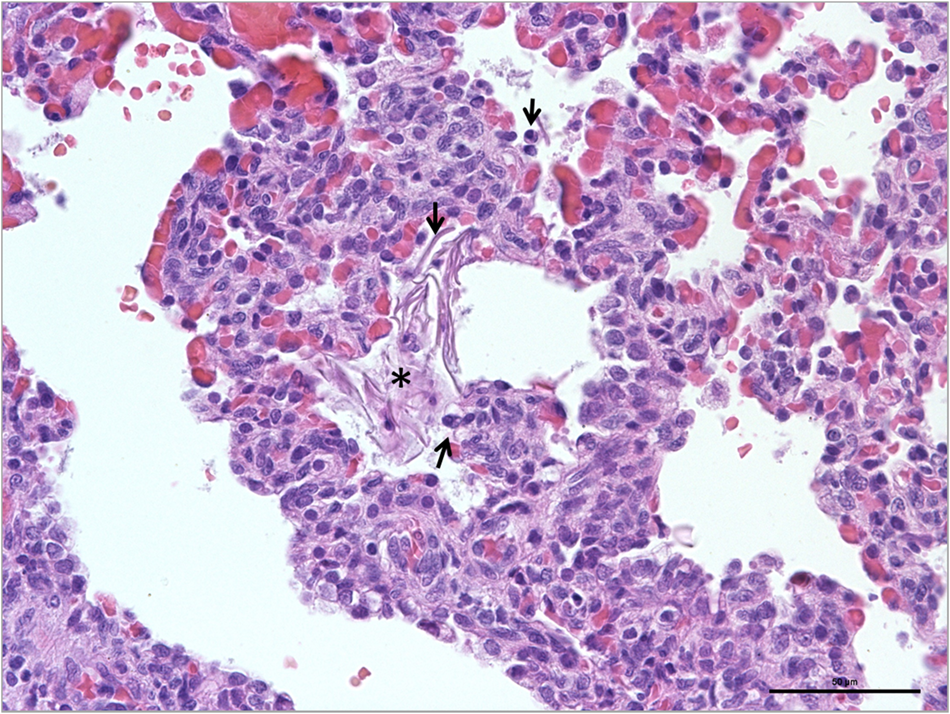
Fig. 5. Aspiration pneumonia, lungs, newborn dog. Presence of a small cluster of epithelial squama (star) in the alveolar lumen. Note that the surrounding lung parenchyma showed diffuse marked capillary congestion and inflammatory cells (arrows; HE; × 400; scale: 50 μm).
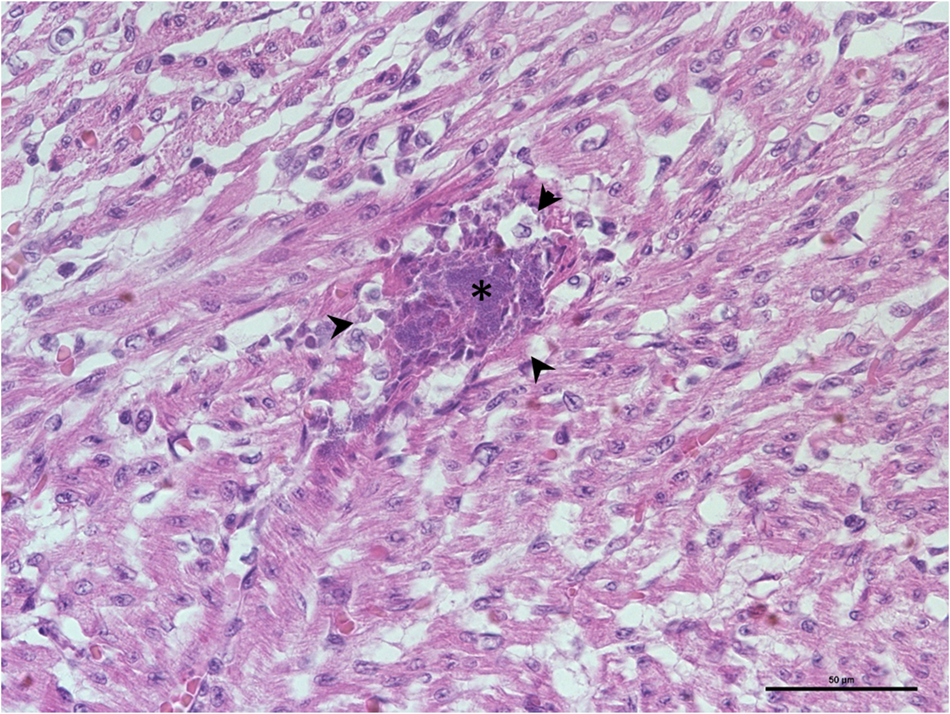
Fig. 6. Myocarditis, heart, newborn dog. Presence of focal basophilic bacterial colonies in the myocardium (star) associated with minimal surrounding inflammatory infiltration (arrows' heads; HE; × 400; scale: 50 μm).
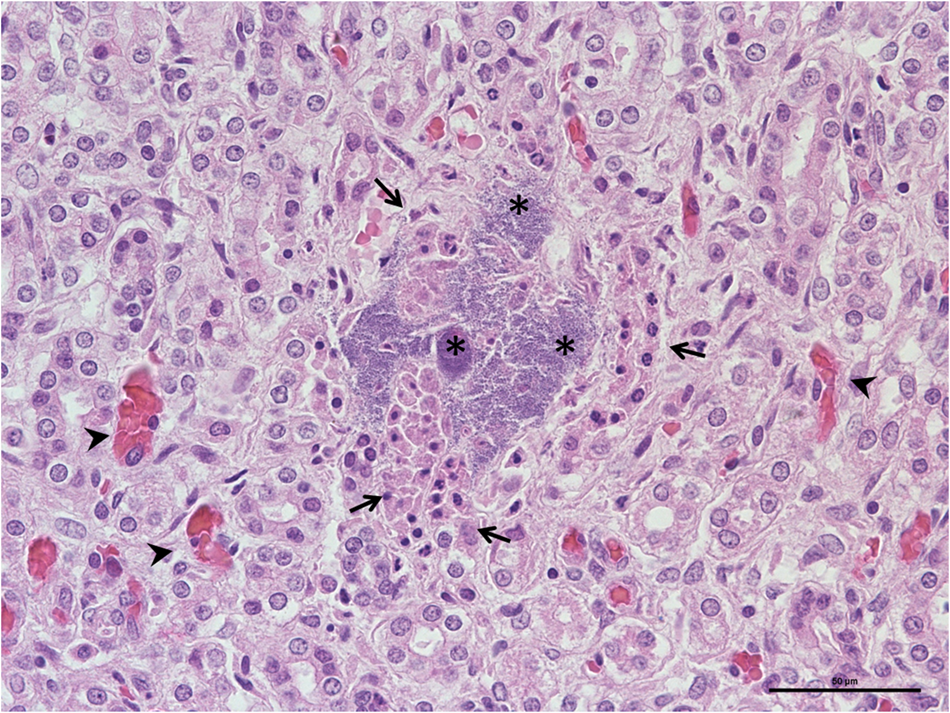
Fig. 7. Nephritis, kidneys, newborn dog. Basophilic bacterial colonies (stars) associated with tubular epithelial degeneration (arrows), hyperemia (arrows' heads) and minimal inflammation (HE; × 400; scale: 50 μm).
Bacteriology
About 80% of newborn puppies who die in the first 3–4 weeks after birth show positive bacteriological results on the postmortem examination (Meloni et al., Reference Meloni, Martino, Grieco, Pisu, Banco, Rota and Veronesi2014; Münnich and Küchenmeister, Reference Münnich and Küchenmeister2014; Guerard, Reference Guerard2019). However, these results are difficult to interpret because many of the bacteria isolated are essentially commensal, such as E. coli (40–60% of cases), Streptococcus spp. (13–47%) or Staphylococcus spp. (7–27%). In 16–47% of cases, several bacteria are isolated from the same sample. Strict pathogens, such as Brucella canis, are more rarely identified (de Souza et al., Reference de Souza, de Carvalho, da Mol, Lopes, Silva, da Paixão and Santos2018). Bacteriological results on the lungs are the most difficult to interpret, as the samples are often contaminated with saliva; bacteria from the oral cavity were found in 50% of lung samples taken from human cadavers (Tsokos, Reference Tsokos2007). Thus, positive bacteriology does not necessarily mean that the bacteria were the cause of clinical disease or death of the animal. Indeed, a positive result can be a true positive with the development of bacteria in the blood before death (in case of sepsis) or false positive which may occur following the agonic spread of bacteria from mucous surfaces, a postmortem translocation linked to the putrefaction process or due to contamination of the sample during collection. Thus, in human medicine, only pure culture is considered a true positive while all mixed results (several different bacterial isolates) are considered false positives or questionable results (Morris et al., Reference Morris, Harrison and Partridge2007). Based on human medical data, administration of antibiotics before death has no significant effect on the results of postmortem bacteriological analyses. Whatever the result obtained (pure culture or mixed culture), the interpretation should always be done in conjunction with other postmortem observations.
PCR
The advantage of PCR is its high sensitivity and thus the ability to demonstrate the presence of even a very small amount of the infectious agent. However, the bacterial and/or viral loads found thanks to PCR are important to take into consideration, as weak positive results may indicate the circulation of the pathogen in the kennel, while a quantitative result with heavy loads is most likely related to infection. The thresholds for differentiating a sick animal from a carrier animal are specific for each laboratory (related to differences in assay methods) and they should be communicated with the results. One of the viruses most often looked for in newborn puppies is canine herpesvirus. Seroprevalence, reflecting the circulation of the virus, is frequent (between 30 to 100% of kennels), depending on the region (Infectious Diseases of the Dog and Cat—4th Edition, n.d.), but the mortality in puppies born to seropositive mothers in a positive breeding kennel is not systematic. It is therefore essential to know the viral load before establishing the etiological diagnosis of death. In cases of doubt, it is possible to ask the laboratory for an opinion on the interpretation of the results.
Coproscopy
Although a positive coproscopy indicates the presence of gastrointestinal parasites, no correlation exists between the intensity of clinical signs and the numbers of diagnostic stages in the fecal sample. On the other hand, a negative result does not necessarily mean that the parasite load is zero. If the clinical suspicion is high, other techniques (e.g. PCR, serology, fecal immunoassays) should be performed to test the clinical hypothesis. In cases of toxocariasis or ancylostomiasis in very young dogs (neonates), coroscopy will probably be negative, as the clinical disease can precede patency defined as the presence of nematode eggs in feces of these young hosts (Buckle et al., Reference Buckle, Hardcastle, Scott, Craig, French, Gedye and Collett2019). Apart from intestinal lesions, some parasites can cause damage to other internal organs. Toxocara canis, present in 22% of fecal samples from pre-weaning puppies (Grellet et al., Reference Grellet, Chastant-Maillard, Robin, Feugier, Boogaerts, Boucraut-Baralon, Grandjean and Polack2014), can cause death from bronchopneumonia or hepatitis in puppies as early as a few days after birth. The parasite migrates from tissues of the dam to her puppies in utero, contributing to the bacterial translocation, in particular to the lungs, being a potential cause of bacteremia, and maturing in the small intestines of puppies postpartum (Buckle et al., Reference Buckle, Hardcastle, Scott, Craig, French, Gedye and Collett2019). Table 6 presents parasitic diseases possible to diagnose in the newborn dog.
Stage 6: diagnosis
In puppies, the combination of various previously cited postmortem examinations allows the diagnosis in more than 90% of cases. Whereas, histopathology and autopsy alone allow the diagnosis to be made in 50% of cases (Guerard, Reference Guerard2019). No pathological or histological findings may be indicative, as in case of sepsis, when the fast course of the disease occurs before visible tissue damage.
In human medicine, a postmortem diagnosis is made at the three following levels (Hanzlick, Reference Hanzlick2006):
• Identification of the immediate cause of death – this is the final consequence of the underlying cause of the disease, an injury or complications that occurred directly before death and resulted in death (e.g. pneumonia).
• Identification of the intermediate cause of death – includes the most important diseases, conditions, or complications that have arisen between the underlying and immediate causes of death (e.g. aspiration of amniotic fluid).
• Identification of the underlying cause of death (or main cause of death) – corresponds to the condition or injury that caused the morbid events leading to death, or the circumstances that led to a fatal injury. Death cannot have occurred without it and it must be as specific as possible (e.g. dystocia).
Identifying the underlying cause of death can help prevent mortality in siblings or in subsequent litters, so it is important to contiune investigation of the immediate cause of death and to identify, ideally, the underlying cause. In the example given above, dystocia could have provoked the aspiration of amniotic fluid and consequently pneumonia – the immediate cause of death. It would therefore be essential to avoid dystocia in future parturition by monitoring the dam, or even to perform an elective C-section in some cases.
Table 7 groups together several examples of analyses of cases of mortality in newborn puppies, the results of various additional examinations, and the conclusions concerning the causes of death.
Table 7. Examples of postmortem examination findings and diagnosis with the lesion table observed in newborn puppies (Buckle et al., Reference Buckle, Hardcastle, Scott, Craig, French, Gedye and Collett2019; Guerard, Reference Guerard2019; Piegari et al., Reference Piegari, Cardillo, Alfano, Vangone, Iovane and Fusco2020 and authors' unpublished data)
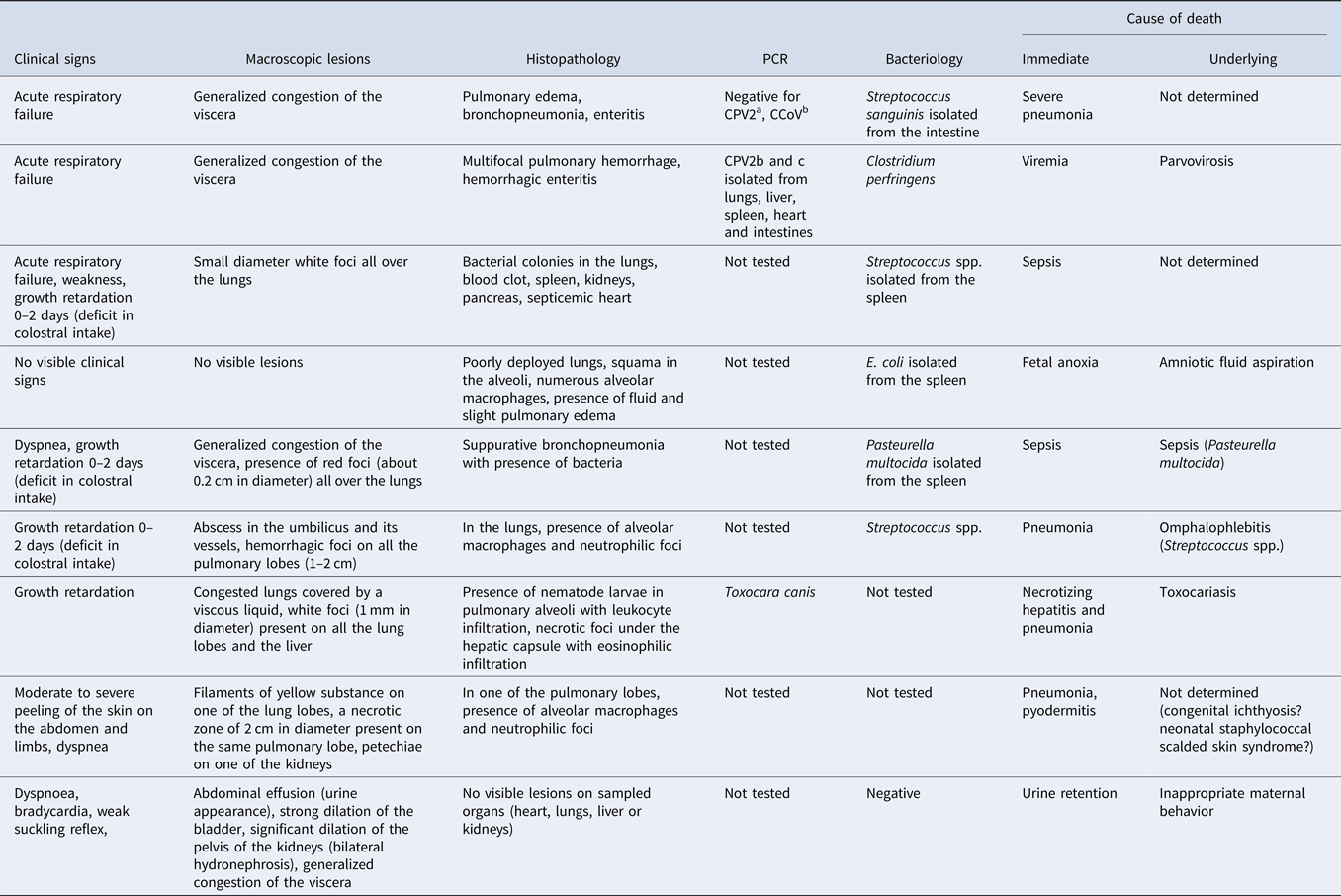
a Canin parvovirus type 2 (CPV2).
b Canin coronavirus (CCoV).
Conclusions
Due to the absence of clinical signs preceding death in the majority of canine newborns, the postmortem examination is often the only examination available for diagnosis. This examination is essential to save the entire litter, and therefore must be as complete as possible. In cases of multiple deaths, it is important to investigate several cadavers, in order to have a global view of the situation in the kennel and to make a diagnosis that is most representative of reality. Indeed, by necropsy of a single cadaver, one can miss a cause of mortality in the kennel.
Acknowledgments
The authors want to thank Prof. Sylvie Chastant-Maillard for writing assistance and general support in the writing of this paper.




















