Introduction
Asthma is the most common non-communicable chronic inflammatory airway disease globally [Reference Jung, Park and Jung1]. It is characterized by airway hyperresponsiveness, inflammation, and long-term airway remodeling with variable airway limitation [Reference Camoretti-Mercado and Lockey2]. Due to its chronicity, it is associated with poor quality of life, increased risk of repeat acute exacerbations, significant morbidity and mortality, and vast healthcare cost burdens [Reference Jung, Park and Jung1,Reference Asher, Rutter and Bissell3]. In 2017, there were an estimated 495,100 deaths from asthma and 22.8 million disability-adjusted life years (DALYs) [Reference Asher, Rutter and Bissell3]. Financially, asthma exacerbations are a major cause of increased healthcare utilization and higher total healthcare costs for patients and society. In 2007, patients with asthma exacerbations spent an estimated $9223 versus $5011 per person per year, with asthma-specific costs of $1740 versus $847 per person per year, compared with patients without exacerbations [Reference Ivanova, Bergman, Birnbaum, Colice, Silverman and McLaurin4]. Total expenditures for asthma in 2007 were estimated to be $56 billion per year with productivity losses due to morbidity and mortality of $3.8 and $2.1 billion, respectively [Reference Castillo, Peters and Busse5,Reference Barnett and Nurmagambetov6]. Furthermore, patients requiring an emergency department (ED) visit or hospitalization for asthma are at significantly increased risk for future exacerbations, exposing an ongoing need to prevent these exacerbating events [Reference Castillo, Peters and Busse5].
Most commonly, asthma exacerbations are triggered by certain environmental exposures. The most common viral-associated infection is human rhinovirus, with hospital admission rates for asthma exacerbations in school-aged children and adults correlating with seasonal peaks in rhinovirus infections [Reference Johnston, Pattemore and Sanderson7]. Other acute triggers include bacterial infections, environmental allergens, and air pollutants such as tobacco smoke, ozone, and particulate matter [Reference Eisner, Klein, Hammond, Koren, Lactao and Iribarren8]. Prevention of exacerbations has been linked to mitigating exposures and triggers, and tailored pharmacologic therapy. The Global Initiative for Asthma (GINA) guidelines were developed and published in 1995 as a collaborative effort between the World Health Organization (WHO) and USA’s National Heart, Lung, and Blood Institute with a goal of (1) translating evolving science on asthma into recommendations for the management and prevention of asthma and (2) to stimulate the implementation and evaluation of practical guidelines in order to reduce the global burden of asthma [Reference Bousquet, Clark and Hurd9].
Following GINA guidelines, as-needed usage of short-acting beta-agonists (SABAs) or inhaled budesonide/formoterol combinations are the first-line treatment for patients with mild intermittent asthma and have been the recommended rescue medication for rapid symptom relief [Reference Papi, Blasi, Canonica, Morandi, Richeldi and Rossi10]. As symptoms worsen, increased doses of scheduled combination therapies are utilized with monoclonal antibodies as a consideration in the most severe cases.
While these pharmacologic therapies are effective, modifiable risk factors involved in the development of acute asthma exacerbations such as obesity and exposure to cigarette smoke are clinically important targets for improving asthma outcomes. An enlarging body of evidence suggests obesity negatively impacts physiologic parameters in asthma [Reference Arismendi, Bantulà, Perpiñá and Picado11]. It is also widely known that cigarette use increases morbidity and mortality in asthma. However, evidence suggests obesity and indoor pollutants such as cigarette smoke may act synergistically, meaning the sum is greater than the added parts, with obesity to increase the likelihood of having asthma as well as symptomatic wheezing [Reference Wu, Brigham and Peng12,Reference Wong, Forno and Celedón13]. Nevertheless, this interaction has not been tested using serum biomarkers to assess for cigarette usage which probed researchers to request evidence of this complex and synergistic interaction between serum biomarkers for cigarettes, obesity, and asthma outcomes [Reference Wong, Forno and Celedón13]. Thus, our objective was to assess the impact of obesity and smoking on asthma exacerbations, ED usage, and hospitalizations with serum cotinine levels, a metabolite of cigarette use. We hypothesized that increased serum cotinine and the presence of obesity would significantly worsen asthma outcomes compared to either variable in isolation.
Methods
Materials
A cross-sectional analysis of the 2011–2015 iterations of the National Health and Nutrition Examination Survey (NHANES) was performed. NHANES is a nationally representative, publicly available database that collects data from non-institutionalized US citizens annually. It is composed of a patient-reported medical questionnaire, physical exam performed by licensed clinicians, and serum laboratory sample collection. Consent was obtained prior to data collection, and resulting information was de-identified prior to being made publicly available. Institutional review board approval was provided by the National Center for Health Statistics.
Subjects
Study inclusion criteria included patients who had a current diagnosis of asthma, age 18 years or greater, provided serum cotinine samples, and completed the physical exam. Persons who had inadequate serum cotinine samples or did not have asthma outcomes documented were excluded. In return for participation, travel expenses and childcare were reimbursed. Data extracted included sociodemographics, serum cotinine levels, body mass index, and asthma outcomes. Measurable asthma outcomes were self-reported and included the following: having had an asthma attack, ED visits for asthma, or hospital visits for any reason in the past 12 months.
Statistical Analysis
Serum cotinine levels were denoted as a categorical variable based on published cotinine cut points with higher than 95% sensitivity and 90% specificity based on race/ethnicity and sex [Reference Tompkins, Beltran and Bedno14]. The cut points varied based on sex, race, and ethnicity. The following cotinine cut points were used: White male (3.26 ng/mL), Black male (7.18 ng/mL), Hispanic male (0.91 ng/mL), White female (5.13 ng/mL), Black female (14.9 ng/mL), and Hispanic female (0.77 ng/mL). Persons with a BMI of 30 kg/m2 or greater were classified as having obesity. Logistic regression models were developed to assess the impact of serum cotinine and obesity on asthma outcomes. The interaction term between elevated cotinine and obesity was created by multiplying the adjusted effect sizes. Survey design and MEC weightings, provided by NHANES, were used to correct for population-level estimates (N). MEC weightings adjust for complex survey design (oversampling of certain groups), survey nonresponse, and post-stratification adjustments to ensure the calculated estimates are representative of US citizens. Statistical analysis was performed using Stata 16.1 (StataCorp, College Station, TX) in June 2022. Synergy was evaluated mathematically and statistically and, for the purposes of this research, indicates the sum is greater than the added parts (i.e. for A + B = C, the effect is additive if C = A + B, but is synergistic if C > A + B).
Results
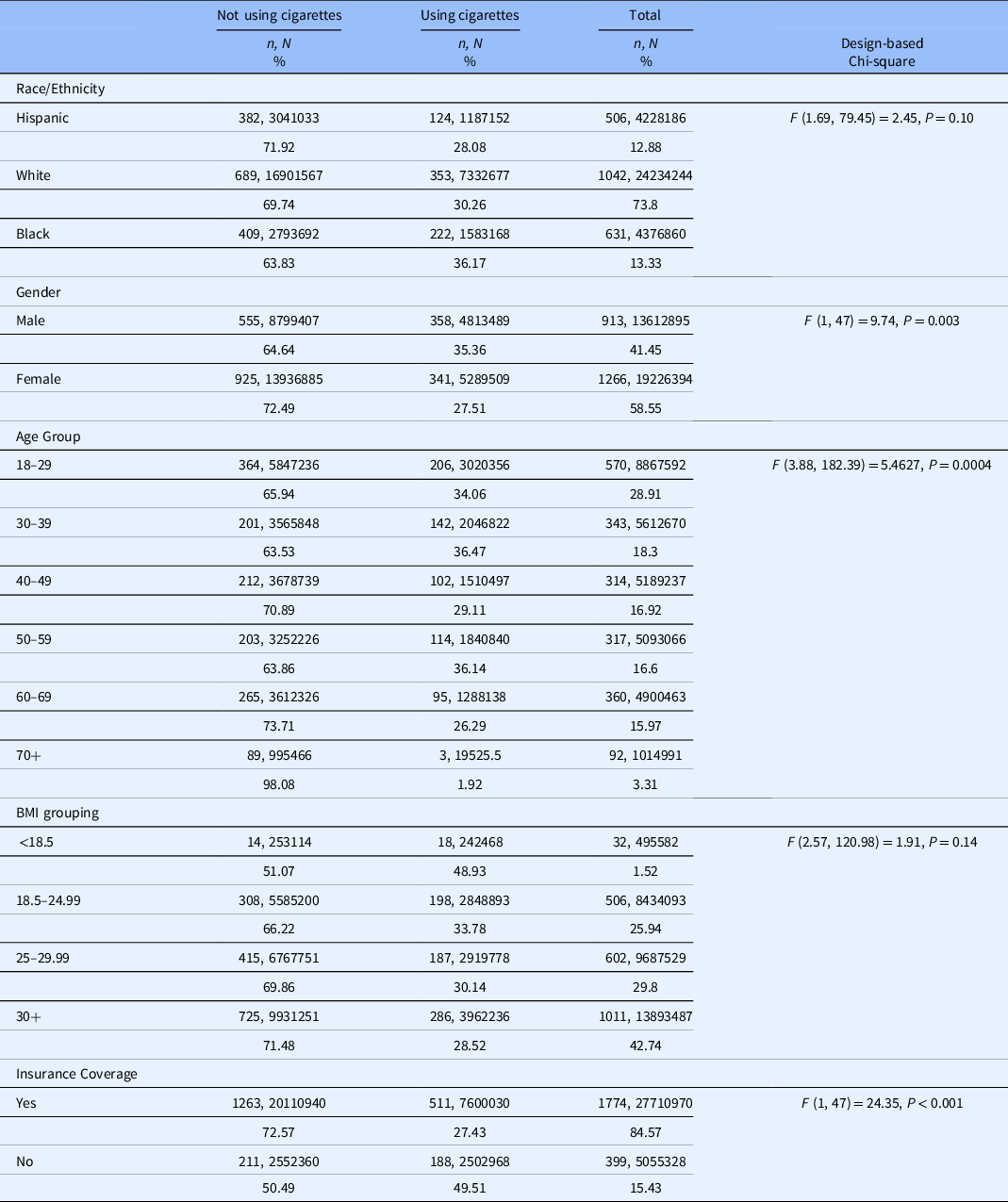
We identified a sample of 2,179 (N = 32,839,290) individuals who ever had asthma. Among this group, males were most likely to currently smoke cigarettes (35.36%; n = 358, N = 4,813,489) as were Black patients (36.17%; n = 222, N = 1,583,168) followed by White patients (30.26%; n = 353, N = 7,332,677) as shown in Table 1. Persons aged 30–39 years were most likely to currently smoke cigarettes (36.47%; n = 142, N = 2,046,822). The majority of patients had healthcare insurance (84.57%). Finally, persons with obesity and asthma represented 42.74% (n = 1011, N = 13,893,487) of the sample.
Table 1. Demographics characteristics by cigarette use among individuals ever having asthma
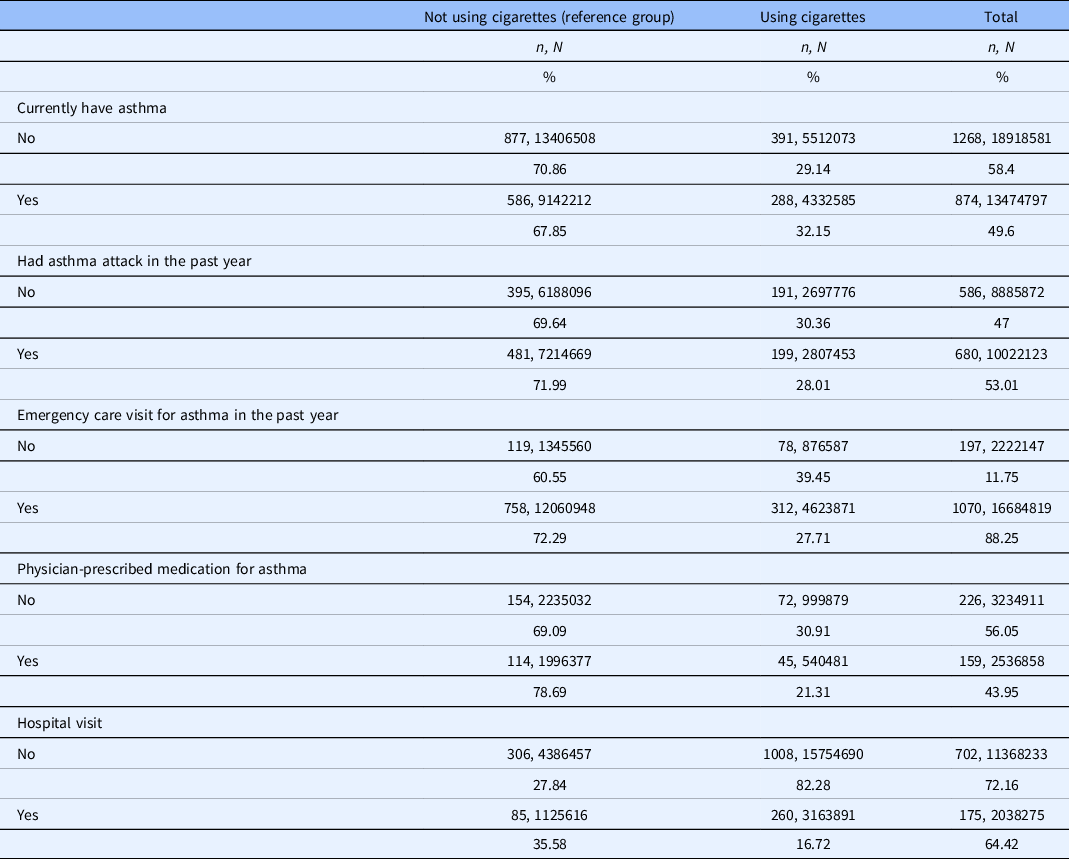
In patients with a current diagnosis of asthma, 32.15% actively smoke cigarettes (n = 288, N = 4,332,585). Among those who currently have asthma and smoke, 28.01% reported having an asthma attack in the past year and 27.71% had an ED visit for asthma in the past year (Table 2). Twenty-one percent of patients with asthma and who currently smoke had a physician-prescribed asthma medication (compared to 78.69% in nonsmokers) and 16.72% were hospitalized overnight for any reason over the previous year.
Table 2. Measured variables among persons with and without cigarette use
Utilizing logistic regression models, persons who had elevated cotinine were significantly more likely to have had an ED visit for asthma in the past year (AOR 1.82; 95% CI 1.19–2.79), have a physician-prescribed asthma medication (AOR 2.04; 95% CI 1.11–3.74), and have a hospitalization for any reason (AOR 3.65; 95% CI 1.88–7.07) compared to patients with low cotinine in the adjusted models (Table 3). Likewise, patients who were obese were more likely to have had an asthma-related ED visit (AOR 1.67; 95% CI 1.06–2.62) or hospitalization for any reason in the past year compared to non-obese patients [AOR 2.76; 95% CI 1.69–4.5 (Table 3)]. While a significant interaction between elevated cotinine and obesity was detected among patients who actively have asthma compared to those who previously had asthma (AOR: 1.76; 95% CI 1.10–2.82), there were no significant interactions between the two dependent variables among patients with asthma that visited an ED for asthma, had an asthma-specific medication, or had an asthma attack in the past year. Interestingly, after applying the interaction term, persons with elevated cotinine and obesity were 62% less likely to have an overnight hospital stay for any reason in the past year (AOR .38; 95% CI 0.19–0.76).
Table 3. Associations between asthma symptomology and serum cotinine and obesity category

A. Among individuals ever having a diagnosis of asthma. B. Among individuals still having asthma. Adjusted models controlled for age, gender, obesity status, cotinine cutoff, race/ethnicity, and insurance coverage. AOR: adjusted odds ratio; BMI: body mass index.
Discussion
We performed a cross-sectional analysis of NHANES, a nationally representative survey, to assess for a synergistic impact between clinical asthma outcomes and increased serum cotinine and obesity. While previous literature has identified an association between these risk factors and asthma outcomes in isolation, this is the first study, to our knowledge, that assesses the interactions of cotinine and obesity on clinical outcomes. Notably, patients with asthma and increased serum cotinine and obesity in isolation had an increased risk of asthma-specific ED usage and hospitalizations for any reason. Persons with increased cotinine and obesity at the same time were more likely to still have asthma, suggesting a synergistic interaction. However, no significant interaction was detected between cotinine and obesity among patients with ED usage, asthma prescriptions, or hospitalizations. These findings provide further support for the deleterious effects of smoking and obesity on asthma-related ED usage and healthcare utilization.
Serum cotinine has a dose-dependent relationship with cigarette exposure and would, therefore, be expected to be more useful than self-reported measures of smoking as a result of social desirability bias or response bias [Reference Rapp, Alpert, Flores and Taioli15]. While it is known that cigarette smoke increases the morbidity of asthma, the present study did not identify a significant interaction effect between obesity, serum cotinine, and asthma outcomes. This synergistic effect was previously suggested by Wu et al. [Reference Wu, Brigham and Peng12] and Wong et al. [Reference Wong, Forno and Celedón13] Wu et al. analyzed data from two cohort studies among children with asthma and noted that urine cotinine and in-home airborne nicotine were both correlated with increased self-reported wheezing and trouble breathing. Wong et al. performed a review of current literature among asthma interactions between obesity and indoor/outdoor pollutants (including cigarette smoke) and noted increased reports of asthma symptoms in the presence of all of the mentioned factors. While a positive interaction existed in likelihood of still having asthma among persons with asthma, obesity, and elevated cotinine, it is possible that this effect does not extend to ED usage and asthma-related hospitalizations. Rather, the effect is isolated to only having self-reported wheezing and/or trouble breathing as the previous studies posited.
Interestingly, we identified a lower likelihood of having an overnight hospital stay for any reason among persons with asthma and elevated cotinine and obesity, after adjusting for interactions. We believe that this may be the result of several factors. First, this regression assessed hospitalizations for any reason, rather than for asthma specifically, and may have been impacted by comorbidities that were not controlled. The presence of these other comorbidities may have increased the frequency at which these patients had primary care appointments which helped reduce hospitalizations [Reference Hu, Dattani and Cox16]. Another potential reason for this unexpected finding could be related to stigma. For instance, patients who have both increased serum cotinine and obesity may experience greater stigma than patients with increased cotinine or obesity in isolation which may have resulted in them being less likely to present to the hospital for evaluation. This is supported by the fact that smokers often delay or avoid medical-seeking behaviors as a result of stigma; a finding replicated among obese patients [Reference Carter-Harris17,Reference Drury and Louis18]. Additionally, misclassification of asthma case definitions may have occurred as survey-based analyses are far from ideal and this survey did not incorporate lung function or asthma severity. Finally, disease severity – which was not possible to accurately measure in this study – may have impacted the interaction between cotinine and obesity regarding hospitalizations.
Potential mechanisms underlying the deleterious effects on asthma outcomes by elevated cotinine and obesity are related to the pro-inflammatory cytokines associated with each condition. Obesity and cigarette exposure independently activate nuclear factor kappa B, a transcription factor widely recognized to induce inflammation in the lungs [Reference Feng, Lu and Ou19,Reference Yang, Chida and Bauter20]. Furthermore, obesity increases pro-inflammatory cytokines IL-6, IL-13, IL-17A, and TNF-α [Reference Dietze, Böcking, Heverhagen, Voelker and Renz21–Reference Dinger, Kasper and Hucklenbruch-Rother23]. Interestingly, IL-6, IL-17A, and TNF-α are also increased after cigarette smoke exposure, further supporting the potential for a combined interaction between cigarette exposure and obesity on airway hyper-reactivity [Reference Siew, Wu, Ying and Corrigan24,Reference Crotty Alexander, Shin and Hwang25].
As a result of the negative health contributions from cigarette use and obesity, public health efforts to reduce their effects are paramount. The most efficacious public health efforts utilize a multimodal approach at the individual, community, and legislative levels. For instance, excise taxation on cigarettes, a result of legislation, has been highly effective at reducing tobacco use in both Africa and the United States [Reference Ho, Schafferer, Lee, Yeh and Hsieh26,Reference Apollonio, Dutra and Glantz27]. Likewise, excise taxes have also been proposed as a method for reducing sugary beverage and alcohol consumption [Reference Chaloupka, Powell and Warner28], both of which contribute to obesity. At the community level, local leaders have made significant improvements in obesity reduction by utilizing interventions that increase school-based physical activity [Reference Yuksel, Şahin, Maksimovic, Drid and Bianco29], physical activity utilizing smartphone interventions [Reference Kim and Seo30], and diet [Reference Trude, Surkan, Cheskin and Gittelsohn31]. Furthermore, the connection between food deserts and obesity is widely recognized with new literature suggesting community involvement, opposed to commercially driven, in supermarket interventions is key to improving these deserts [Reference Brinkley, Glennie, Chrisinger and Flores32]. Finally, public health efforts at the individual level are also useful in reducing obesity and smoking, both of which have been studied in counseling sessions [Reference Raman, Hay, Tchanturia and Smith33,Reference Hartmann-Boyce, Livingstone-Banks and Ordóñez-Mena34].
Our study had several strengths and limitations. First, while laboratory specimens were objective measures, ED usage and hospitalizations were self-reported which may have imputed response bias into our analyses. Additionally, the presence of asthma was self-reported, which is not as reliable as a physician’s diagnosis of asthma, and may have impacted our findings. The lack of objective lung function measurements also made quantification of asthma severity impossible. This was also a cross-sectional study which prevented the deduction of correlations and only allows for associations to be ascertained. We were also unable to identify cotinine levels by first-hand compared to second-hand smoking exposure. Finally, our exclusion of participants that did not have a serum cotinine value may have confounded our findings. The complex sampling methodology and large sample size with representation across the United States were notable study strengths.
This study identified elevated serum cotinine and obesity as key risk factors for poor asthma outcomes which has been previously understudied. Although we did not identify a synergistic interaction between serum cotinine and obesity on asthma outcomes, the present study highlights the potential usefulness for serum cotinine in clinical practice, particularly when risk-stratifying patients who are highly suspected of smoking nicotine or may be exposed to second-hand smoke in varying degrees. Further research should assess the longitudinal effects of elevated serum cotinine in the setting of patients who have asthma and are obese. Additionally, further research should assess the interaction between risk of hospitalization and concomitant elevation of serum cotinine and obesity.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Dr. Greiner is supported by training grant T32 AI155385 from the U.S. National Institutes of Health. Dr. Hartwell receives grant funding through the National Institute of Justice and Health Resources Services Administration. The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.





