Introduction
Cape Verde is an Atlantic and volcanic archipelago that lies c. 570 km west of Senegal on the African coast. Since the publication of a critical checklist of lichens and allied fungi of the Cape Verde Islands by Mies (Reference Mies1993), considerable progress has been made in the study of its lichen flora. New species have been described from the archipelago in the genera Amandinea, Buellia (Elix & van den Boom Reference Elix and van den Boom2022), Caloplaca (Arup & van den Boom Reference Arup and van den Boom2011), Cratiria (Elix & van den Boom Reference Elix and van den Boom2022), Dirina (Tehler et al. Reference Tehler, Ertz and Irestedt2013), Lecidella (Knoph & Mies Reference Knoph and Mies1995), Llimonaea (Egea et al. Reference Egea, Torrente and Mies1995), Plectocarpon (Ertz & van den Boom Reference Ertz and van den Boom2012), Rinodina (Giralt & van den Boom Reference Giralt and van den Boom2008) and Thelopsis (van den Boom Reference van den Boom2012). Many other additional species have been newly reported for the archipelago (e.g. Büdel & Mies Reference Büdel and Mies1993; Tehler et al. Reference Tehler, Dahlkild, Eldenäs and Feige2004; Llop & van den Boom Reference Llop and van den Boom2009; van den Boom Reference van den Boom2012; Ertz & van den Boom Reference Ertz and van den Boom2020; Zhurbenko et al. Reference Zhurbenko, Diederich and Gagarina2020).
The arid climate with salinic conditions through aerosol salts favours a diverse lichen vegetation dominated by Arthoniales (Fig. 1). Species of Dirina, Lecanographa and Roccella are abundant on coastal and mainly north-east exposed rocks when reached by maritime salt spray, blown in or uplifted by the trade winds, sometimes to rather high altitudes (c. 1000 m elev.) when the topography of the islands is without any high ascending interruption (Mies Reference Mies1993). But the most peculiar Arthoniales is the Cape Verdean endemic Gorgadesia mira Tav., remarkable in its fruticose thallus with lirelliform-dendroid ascomata (Tavares Reference Tavares1964; Follmann & Mies Reference Follmann and Mies1986) (Fig. 1).

Fig. 1. North-east coast of São Vicente between Baía das Gatas and Calhau, with large cliffs in arid and salinic conditions that favour a diverse lichen vegetation dominated by Arthoniales, including Gorgadesia mira, Ingaderia dendritica, I. flexuosa and Sparria caboverdensis. In colour online.
A study of recent collections made by the authors in Cape Verde revealed several undescribed crustose Arthoniales. The aim of this study is to describe these new species. A phylogeny of the Opegraphaceae is provided, and the phylogenetic positions of the type species of the genus Llimonaea and of Fulvophyton sorediatum (Sparrius et al.) Tehler & van den Boom are revealed. The new molecular data result in an enlarged concept of the genus Ingaderia.
Materials and Methods
Voucher specimens are deposited in the herbaria BR, FR, M and S. The external morphology was studied and measured using an Olympus SZX12 stereomicroscope. Macroscopic images were captured with a Keyence VHX-5000 digital microscope and a VH-Z20R/W/T lens. Hand-cut sections and squash preparations of thalli were mounted in water, a 5% aqueous potassium hydroxide solution (K), or in Lugol's iodine solution (1% I2) without (I) or with K pretreatment (KI), and studied using an Olympus BX51 compound microscope. Measurements of ascospores do not include the perispore (=gelatinous sheath) and are reported as (minimum–) ![]() $(\bar{x}- \hbox{SD})$ –
$(\bar{x}- \hbox{SD})$ – ![]() $(\bar{x} + \hbox{SD})$ (–maximum), followed by number of measurements (n), and the values are rounded to the nearest 0.5 μm. Measurements refer to dimensions in water. Microscopic images were captured using an Olympus BX51 compound microscope fitted with an Olympus SC50 digital camera. Colour reactions of the thallus were studied using K, common household bleach (C), K followed by common household bleach (KC), crystals of para-phenylenediamine dissolved in ethanol (PD) and long-wave UV (366 nm). Lichen secondary metabolites were investigated using thin-layer chromatography (TLC) in solvents EA and G (Orange et al. Reference Orange, James and White2010).
$(\bar{x} + \hbox{SD})$ (–maximum), followed by number of measurements (n), and the values are rounded to the nearest 0.5 μm. Measurements refer to dimensions in water. Microscopic images were captured using an Olympus BX51 compound microscope fitted with an Olympus SC50 digital camera. Colour reactions of the thallus were studied using K, common household bleach (C), K followed by common household bleach (KC), crystals of para-phenylenediamine dissolved in ethanol (PD) and long-wave UV (366 nm). Lichen secondary metabolites were investigated using thin-layer chromatography (TLC) in solvents EA and G (Orange et al. Reference Orange, James and White2010).
Molecular techniques
Well-preserved and freshly collected specimens (less than two months) or specimens kept in a freezer and frozen less than two months after collection and lacking any visible symptoms of fungal infection were used for DNA isolation. Genomic DNA was isolated from lichen specimens using the CTAB extraction protocol (Doyle & Doyle Reference Doyle and Doyle1990). For three specimens (Llimonaea flexuosa Ertz 17273, L. occulta Ertz 16919 and L. sorediata Ertz 17030), hand-cut sections of the ascomata were used for direct PCR as described in Ertz et al. (Reference Ertz, Tehler, Irestedt, Frisch, Thor and van den Boom2015). The material was then added to a tube containing the PCR reaction mixture and amplified directly. Amplification reactions were prepared for a 50 μl final volume containing 5 μl 10× DreamTaq buffer (Fermentas), 1.25 μl of each of the 20 μM primers, 5 μl of 2.5 mg ml−1 bovine serum albumin (Fermentas #B14), 4 μl of 2.5 mM each dNTPs (Fermentas), 1.25 U DreamTaq DNA polymerase (Fermentas) and 1 μl of template genomic DNA or tiny fragments of lichen material. A targeted fragment of c. 1 kb of the RPB2 protein-coding gene was amplified using the primers fRPB2-7cF and fRPB2-11aR (Liu et al. Reference Liu, Whelen and Hall1999), and a fragment of c. 1.4 kb at the 5ʹ end of the nuLSU rDNA was amplified using primers LIC15R (Miadlikowska et al. Reference Miadlikowska, McCune and Lutzoni2002) and LR7 (Vilgalys & Hester Reference Vilgalys and Hester1990). Cycling conditions for RPB2 included initial denaturation at 95 °C for 3 min (genomic DNA) or for 10 min (direct PCR), 35 cycles of 95 °C for 45 s, 52 °C for 1 min and 72 °C for 1 min, with a final extension step at 72 °C for 10 min. Cycling conditions for nuLSU included initial denaturation at 95 °C for 3 min (genomic DNA) or for 10 min (direct PCR), 25 cycles of 95 °C for 45 s, 52 °C for 40 s and 72 °C for 2.5 min, 14 cycles of 95 °C for 45 s, 52 °C for 40 s and 72 °C for 2.5 min (+5 s per cycle), with a final extension step at 72 °C for 10 min. Both strands were sequenced by Macrogen® using amplification primers, and with the additional primers LR3R and LR3 (Vilgalys & Hester Reference Vilgalys and Hester1990) for nuLSU. Sequence fragments were assembled with Sequencher v. 5.4.6 (Gene Codes Corporation, Ann Arbor, Michigan, USA). Sequences were subjected to MegaBLAST searches to verify their closest relatives and to detect potential contaminations.
Taxon selection and phylogenetic analyses
The newly generated sequences were subjected to NCBI BLAST searches (https://www.ncbi.nlm.nih.gov) in order to obtain a first approximation of phylogenetic affiliation. A two-locus dataset (nuLSU and RPB2) was assembled for placing the newly sequenced taxa in a phylogeny of the Opegraphaceae as circumscribed by Ertz & Tehler (Reference Ertz and Tehler2011). Sequences published in Tehler & Irestedt (Reference Tehler and Irestedt2007), Ertz et al. (Reference Ertz, Miadlikowska, Lutzoni, Dessein, Raspe, Vigneron, Hofstetter and Diederich2009), Ertz & Tehler (Reference Ertz and Tehler2011), Frisch et al. (Reference Frisch, Thor, Ertz and Grube2014), Ertz (Reference Ertz2020), Diederich & Ertz (Reference Diederich and Ertz2020) and Perlmutter et al. (Reference Perlmutter, Rivas Plata, LaGreca, Aptroot, Lücking, Tehler and Ertz2020) were retrieved from GenBank. The sequences (Table 1) were aligned using MAFFT v. 7.490 (Katoh & Standley Reference Katoh and Standley2013) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) and manually corrected for errors using Mesquite v. 3.04 (Maddison & Maddison Reference Maddison and Maddison2015). Terminal ends of sequences, ambiguously aligned regions and introns were delimited manually and excluded from the datasets. The resulting matrix of Opegraphaceae consisted of 63 terminals. Three species of Roccellaceae, viz. Dichosporidium brunnthaleri (Zahlbr.) G. Thor, Enterographa crassa (DC.) Fée and Erythrodecton granulatum (Mont.) G. Thor, were selected as the rooting taxa from Frisch et al. (Reference Frisch, Thor, Ertz and Grube2014). The final concatenated alignment consisted of 1731 (828 for nuLSU and 903 for RPB2) unambiguously aligned sites.
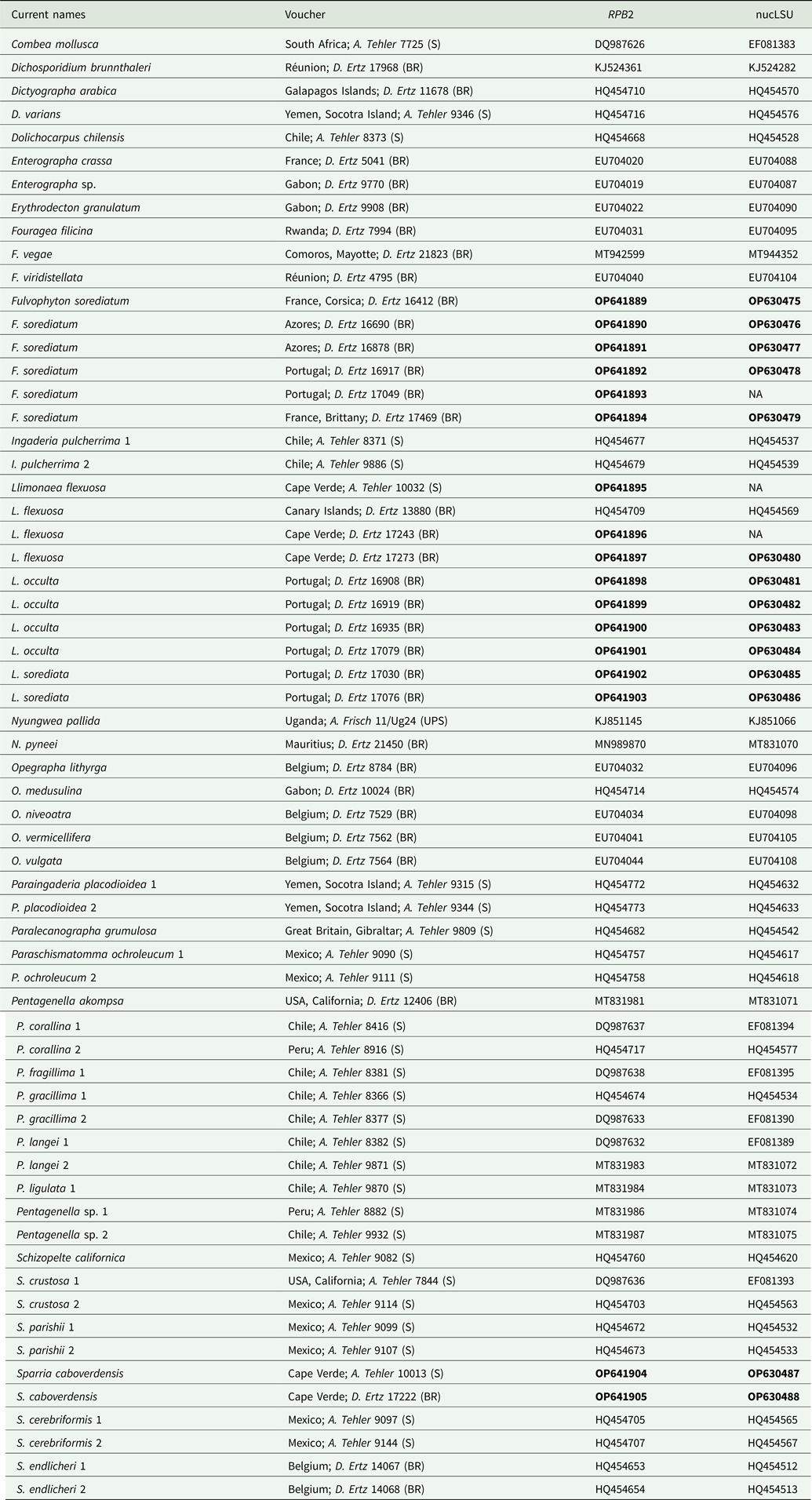
Table 1. Specimens and DNA sequences used in this study, with their respective voucher information. GenBank Accession numbers in bold refer to sequences generated by this project. All other sequences were obtained directly from GenBank.
Best-fit evolutionary models were estimated using the Akaike Information Criterion (AIC) as implemented in jModelTest v. 2.1.6 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012). The TrN + I + G model was selected for the nuLSU dataset, the TIM1 + G model was selected for the RPB2/1st position, the GTR + I + G model for the RPB2/2nd position and the TIM3 + I + G model for the RPB2/3rd position datasets.
Analyses for topological incongruence among loci were carried out by analyzing the single locus datasets with a maximum likelihood (ML) approach using the program RAxML v. 8.2.12 (Stamatakis Reference Stamatakis2014) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). The GTRGAMMA model was used and node support was assessed running 1000 bootstrap replicates. We analyzed the two single-locus datasets for topological incongruence by assuming a conflict to be significant when two different relationships (one being monophyletic and the other non-monophyletic) for the same set of taxa were both supported with bootstrap values ≥ 70% (Mason-Gamer & Kellogg Reference Mason-Gamer and Kellogg1996; Reeb et al. Reference Reeb, Lutzoni and Roux2004). Based on this criterion, no conflict was detected and the nuLSU and RPB2 datasets were concatenated.
Bayesian analyses were carried out on the two-locus datasets under the selected models for four partitions (nuLSU, RPB2/1st, RPB2/2nd and RPB2/3rd positions) using the Metropolis-coupled Markov chain Monte Carlo method (MCMCMC) in MrBayes v. 3.2.7a (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2001; Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010). Two parallel MCMCMC runs were performed each using four independent chains and 40 million generations, sampling trees every 1000th generation. Posterior probabilities (PP) were determined by calculating a majority-rule consensus tree generated from the 60 002 post burn-in trees of the 80 002 trees sampled by the two MCMCMC runs using the sumt option of MrBayes. Tracer v. 1.7.1 (Rambaut et al. Reference Rambaut, Drummond, Xie, Baele and Suchard2018) was used to ensure that stationarity was reached by plotting the log-likelihood values of the sample points against generation time, making sure that the ESS values were much higher than 200. Convergence between runs was also verified using the PSRF (Potential Scale Reduction Factor), where values were all equal or close to 1.000.
In addition, a ML analysis was performed on the two-locus dataset using RAxML v. 8.2.12 (Stamatakis Reference Stamatakis2014) on the CIPRES Web Portal (Miller et al. Reference Miller, Pfeiffer and Schwartz2010) with 1000 ML bootstrap iterations (ML-BS). The two-locus dataset was divided into four partitions (nuLSU, RPB2/1st, RPB2/2nd and RPB2/3rd positions) with the GTRGAMMA model. Phylogenetic trees were visualized using FigTree v. 1.4.2 (Rambaut Reference Rambaut2012).
The ML tree did not contradict the Bayesian tree topology for the strongly supported branches. Therefore, only the ML tree is shown, with the ML-BS values added above the internal branches and the PP values added below the internal branches (Fig. 2). ML-BS ≥ 70 and PP ≥ 0.95 were considered to be significant.

Fig. 2. Phylogeny of Opegraphaceae based on a dataset of nuLSU and RPB2 sequences that resulted from the RAxML analysis. Maximum likelihood bootstrap values are shown above internal branches and posterior probabilities obtained from a Bayesian analysis are shown below. Internal branches, considered strongly supported by both analyses, are represented by thicker lines. The newly sequenced samples are in bold and their names are followed by collection numbers of authors, which act as specimen and sequence identifiers. The lineage corresponding to the enlarged concept of the genus Ingaderia is highlighted, as well as the new species Sparria caboverdensis. In colour online.
Results
Phylogenetic analysis
Thirty-one new sequences (14 nuLSU, 17 RPB2) were obtained for this study and 92 additional sequences (46 nuLSU, 46 RPB2) were retrieved from GenBank, representing a total of 40 species and 63 specimens (Table 1). The RAxML tree obtained from the combined two-locus analysis of the Opegraphaceae dataset is shown in Fig. 2. Relationships within Opegraphaceae are generally well supported, except for the backbone of the tree. The main well-supported lineages are in accordance with the results obtained by Ertz (Reference Ertz2020) and Perlmutter et al. (Reference Perlmutter, Rivas Plata, LaGreca, Aptroot, Lücking, Tehler and Ertz2020). The type of the genus Llimonaea, L. occulta Egea & Torrente, is sequenced for the first time and forms with L. sorediata van den Boom et al. a well-supported lineage sister to the genus Paraingaderia. Fulvophyton sorediatum is the sister species to Llimonaea-Paraingaderia, a relationship that is fully supported. Therefore, that species is well nested within the family Opegraphaceae and does not belong to Fulvophyton in the Roccellographaceae. The genus Llimonaea is recovered as paraphyletic because of the placement of L. flexuosa Egea et al. as sister to a clade including Llimonaea s. str. but also the genera Ingaderia and Paraingaderia, and Fulvophyton sorediatum. Sparria caboverdensis Ertz & Tehler is the sister species to Sparria cerebriformis (Egea & Torrente) Ertz & Tehler and S. endlicheri (Garov.) Ertz & Tehler, a relationship that strongly supports its placement in the genus Sparria. We were unsuccessful with the sequencing of the other new species described from Cape Verde below, mainly because the DNA had not been extracted or the specimens kept in the freezer after collection became too old for sequencing.
Taxonomy
New species
Ingaderia dendritica Ertz & Tehler sp. nov.
MycoBank No.: MB 846082
Differing from Ingaderia flexuosa (Egea et al.) Ertz & Tehler by the narrower (up to 0.4 mm wide), richly dichotomously branched lirellae remaining mostly immersed in the thallus, and by the production of erythrin as a major secondary metabolite.
Type: Cape Verde, São Vicente, Praia Grande on NE-facing ridge above the beach, 16°51.549ʹN, 24°53.195ʹW, 100–200 m elev., on vertical N-facing cliffs, 1 January 2010, A. Tehler 10033 (S—holotype!).
(Fig. 3)

Fig. 3. Ingaderia dendritica (B, D, F & G, Tehler 10033; A, C, E & H, Ertz 17229; I, Ertz 17280). A–C, thallus and ascomata (A taken in the field). D, section through an ascoma in water. E, ascus in KI. F, conidia in K. G–I, ascospores in water, except the second G (right) in K. Scales: A = 1 cm; B & C = 1 mm; D = 100 μm; E–I = 10 μm (G, H and I, same magnification). In colour online.
Thallus crustose, pale creamish, rimose-areolate, mostly flat, smooth to slightly rugulose, matt; areoles 0.3–0.8(–1) mm diam., in section c. 150–250 μm thick; upper cortex inconspicuous or poorly differentiated, c. 12–20 μm thick, of ‘branched type’ (as defined by Aptroot & Schumm (Reference Aptroot and Schumm2011: p. 7)); rich in crystals (polarized light), some dissolving in KOH and others of calcium oxalate (H2SO4!). Photobiont trentepohlioid; cells rounded to elongate, 7–20 × 6–12 μm.
Ascomata immersed in the thallus, becoming slightly elevated above the thallus surface, lirelliform, flexuose, richly dichotomously branched giving a dendritic appearance, remaining narrow, not forming stromatic aggregates, 1–8(–10) × 0.15–0.3(–0.4) mm but regularly divided into shorter fragments corresponding to the thallus areoles, black, mostly covered by a thin layer of thallus c. 5–25 μm thick, often appearing slightly whitish pruinose along the slit-like opening; hymenial disc remaining slit-like. Excipulum dark brown to black, c. 45–95 μm thick, fused with the hypothecium below, K+ becoming slightly darker. Hymenium clear, hyaline to pale fawn, 80–115 μm tall, I+ red, KI+ pale blue; epihymenium hyaline, I+ red, KI+ pale blue. Paraphysoids richly branched-anastomosing, 2–2.5 μm thick, not or slightly enlarged to 3.5 μm in epihymenium. Hypothecium dark brown, thick, extending to the substratum, 125–250 μm thick, I−, K+ becoming slightly darker. Asci (4–)8-spored, narrowly clavate, 80–105 × 17–22 μm, with a distinct ocular chamber; wall not distinctly thicker at apex; endoascus KI+ blue with a KI+ blue apical ring. Ascospores fusiform with the upper half often wider, 3–7(–8)-septate, dark brown from an early stage, with a distinct dark brown verruculose ornamentation on the wall, K+ becoming slightly darker, (17–)20–26.5(–31) × (5–)5–7(–9) μm (n = 35); gelatinous sheath c. 0.5–1.5 μm sometimes present.
Pycnidia immersed in the thallus, visible as dark brown dots, sometimes slightly whitish pruinose, surrounded by a thin whitish to creamish margin, level with the thallus surface and separated from it by a crack, c. 90–190 μm diam.; spherical in cross-section; wall dark brown; conidia hyaline, filiform, slightly curved to sickle-shaped, 13–18 × 1 μm.
Chemistry
Thallus C+ red, K−, UV−. TLC (solvent EA): erythrin (major) (specimens tested: Ertz 17229, 17280 and Tehler 10033).
Etymology
The epithet is named after the dendritic appearance of the ascomata.
Distribution and ecology
So far known only from the island of São Vicente in Cape Verde, where it inhabits volcanic rock of vertical N-facing cliffs in arid and open conditions near the sea.
Discussion
The new species differs from Ingaderia flexuosa (see emended description below) by its narrower (up to 0.4 mm wide vs up to 0.6 mm in I. flexuosa) richly branched-dendroid lirellae remaining immersed in the thallus, becoming only slightly elevated above the thallus surface, and by the production of erythrin. Ingaderia occulta (Egea & Torrente) Ertz also has a thick black excipulum and hypothecium, similar ascospores and produces erythrin. It differs from I. dendritica by the ascomata that are shortly lirellate to roundish, that become elevated, with a slit-like opening which gets wider at maturity (Torrente & Egea Reference Torrente and Egea1991; Egea et al. Reference Egea, Torrente and Mies1995).
Additional specimens examined
Cape Verde: São Vicente: S of the road between Baia das Gates and Calhau, NE of Monte Verde, 16°52ʹ29ʺN, 24°54ʹ46ʺW, c. 200 m elev., on vertical NE-facing coastal volcanic outcrops, on rock, 2011, D. Ertz 17229 (BR); ibid., NW of Calhau, northern slope of Goa Baixo, 16°51ʹ27ʺN, 24°52ʹ42ʺW, c. 120 m elev., strongly sloping and vertical coastal volcanic outcrops, on rock, 2011, D. Ertz 17280 (BR).
Sparria caboverdensis Ertz & Tehler sp. nov.
MycoBank No.: MB 846083
Differing from Sparria cerebriformis (Egea & Torrente) Ertz & Tehler by having smaller stroma-like ascomata 0.5–1.5(–2) mm diam., a narrower hymenium (95–190 μm) and non-muriform ascospores with 3–7 transverse septa.
Type: Cape Verde, São Vicente, Praia da Ceilada do Calhau, 2–3 km NW of Calhau on NE-facing ridge above the beach, 16°51.552ʹN, 24°52.741ʹW, c. 150 m elev., on vertical N-facing cliffs, 30 December 2009, A. Tehler 10013 (S—holotype!).
(Fig. 4)

Fig. 4. Sparria caboverdensis (A & B, D–G, Tehler 10013; C, Ertz 17277). A–C, thallus and ascomata. D, section through an ascoma. E, ascus and paraphysoids in K. F, ascospores in K. G, conidia in water. Scales: A–C = 1 mm; D = 100 μm; E–G = 10 μm. In colour online.
Thallus crustose, pale creamish, rimose-areolate, flat or bullate, smooth to slightly rugulose, matt; areoles 0.3–2 mm diam., in section up to 0.7 mm thick; upper cortex c. 50–90 μm thick, of ‘branched type’ (as defined by Aptroot & Schumm (Reference Aptroot and Schumm2011: p. 7)); rich in crystals (polarized light), some dissolving in KOH and others of calcium oxalate (H2SO4!). Photobiont trentepohlioid; cells rounded to elongate, 11–20 × 8–14 μm.
Ascomata immersed, first lirelliform with short branches or rarely irregularly rounded, becoming ±cerebriform or aggregated into rounded to slightly elongated and elevated stroma-like structures 0.5–1.5(–2) mm diam., surrounded by a thin white thalline margin c. 25–60 μm thick; hymenial disc plane, level with the thallus margin, black, often covered with a thin layer of whitish pruina. Excipulum thin, brownish, c. 10–20 μm, sometimes inconspicuous, K+ becoming slightly darker. Hymenium clear, hyaline to pale fawn, 95–190 μm tall, I+ red, KI+ pale blue, sometimes with strands of hyphae richly covered by hyaline crystals; epihymenium dark brown, c. 13–25 μm thick, I+ red, KI+ pale blue. Paraphysoids richly branched-anastomosing, 2–3 μm thick, slightly enlarged to 4 μm and often pale brownish in the epihymenium. Hypothecium dark brown, extending to medulla or substratum, (40–)150–350 μm thick, I−, K+ becoming slightly darker. Asci (6–)8-spored, clavate, 90–125 × 22–30 μm, with a distinct ocular chamber; wall KI−. Ascospores fusiform with the upper half often wider, 3–7-septate, dark brown from an early stage, with a distinct dark brown verruculose ornamentation on the wall, K+ becoming slightly darker, (23–)26–32(–35) × (7–)7.5–10(–11) μm (n = 26); gelatinous sheath c. 0.5–1 μm sometimes present.
Pycnidia immersed in the thallus, visible as dark brown dots, surrounded by a thin whitish to cream margin, level with the thallus surface and separated from it by a crack, 80–115 μm diam.; spherical in cross-section; wall dark brown; conidia hyaline, filiform, slightly curved to sickle-shaped, 17–22 × 1–1.4 μm.
Chemistry
Thallus C+ red, K−, UV−. TLC (solvents EA and G): erythrin.
Etymology
The epithet is named after the archipelago of Cape Verde, where the new species was collected.
Distribution and ecology
So far known only from the island of São Vicente in Cape Verde, where it inhabits volcanic rock of vertical N- and NE-facing cliffs in arid and open conditions near the sea.
Discussion
The species belongs to the genus Sparria according to our phylogenetic tree (Fig. 2). This generic position is also supported by morphological and chemical data since the new species shares the following with the generic type, S. cerebriformis: a crustose, epilithic, corticate thallus; lirellate and immersed ascomata surrounded by a white rim and forming stroma-like structures; a black hymenial disc with whitish pruina; a thin, dark brown proper exciple; a thick dark brown hypothecium; brown ascospores; the production of erythrin. Sparria cerebriformis differs from the new species by the larger stroma-like structures (1–3 mm diam.), a taller hymenium (300–350 μm) and muriform ascospores. Furthermore, it is known only from the coastal areas of California and Baja California (Egea & Torrente Reference Egea and Torrente1995). The only other species known in the genus Sparria, the European S. endlicheri, differs by having a sorediate thallus and a chemistry with lecanoric acid only (Cannon et al. Reference Cannon, Coppins, Ertz, Fletcher, Pentecost and Simkin2021). The ascomata were very rarely observed in S. endlicheri, with ascospores described as 3–5-septate, 14–20 × 5–7 μm (Cannon et al. Reference Cannon, Coppins, Ertz, Fletcher, Pentecost and Simkin2021), thus smaller than in the new species. Sparria caboverdensis differs from Ingaderia flexuosa (Egea et al. Reference Egea, Torrente and Mies1995; sub. Llimonaea flexuosa, description emended in this study, see below) by the different ascomata type (long lirelliform and never forming stroma-like structures in I. flexuosa), a much thinner excipulum (60–100(–150) μm in I. flexuosa), much wider ascospores (4–6 μm in I. flexuosa) and the production of erythrin as secondary thallus metabolite (gyrophoric acid often with a trace of lecanoric acid in I. flexuosa).
Additional specimens examined
Cape Verde: São Vicente: NW of Calhau, northern slope of Goa Baixo, 16°51ʹ27ʺN, 24°52ʹ42ʺW, c. 120 m elev., on vertical cliffs, on rock, 2011, Ertz 17277 (BR); ibid., S of the road between Baia das Gates and Calhau, NE of Monte Verde, 16°52ʹ29ʺN, 24°54ʹ46ʺW, c. 200 m elev., on vertical NE-facing cliffs, on rock, 2011, Ertz 17221, 17222 (BR).
Syncesia miesii Tehler & Ertz sp. nov.
MycoBank No.: MB 846084
Differing from Syncesia sulphurea (Vainio) Tehler by an I− thallus, a tomentose hymenial disc, a taller hymenium (85–115 μm) and a different chemistry with fatty acids only (including cf. roccellic acid).
Type: Cape Verde, São Vicente, E of Mindelo, Monte Verde, 16°52ʹ22ʺN, 24°55ʹ58ʺW, c. 670 m elev., sheltered volcanic rocks, 3 December 2011, D. Ertz 17188 (BR—holotype!).
(Fig. 5)

Fig. 5. Syncesia miesii (A, B, D, E & H, Ertz 17188; C, F & G, Ertz 17192). A–C, thallus and ascomata. D, section through an ascoma in water. E, ascus in water. F & G, asci in KI. H, ascospores in water. Scales: A–C = 1 mm; D = 100 μm; E–H = 10 μm. In colour online.
Thallus crustose to somewhat placodioid, creamy white to white brownish, continuous to rimose-areolate, mostly flat, ecorticate, smooth to rugulose, matt, pruinose; areoles 0.2–1 mm diam., in section c. 0.2–0.5(–0.8) mm thick, with a white to white-greyish medulla often becoming dirty brown below; rich in crystals (polarized light), some dissolving in KOH and others of calcium oxalate (H2SO4!). Prothallus ±byssoid, dark brown, 1–2 mm wide. Photobiont trentepohlioid; cells rounded to elongate, (8–)11–23 × 7–13 μm.
Ascomata pluricarpocentral, apothecioid, synascoma absent or poorly developed, circular in outline, first flat and level with the thallus surface, becoming elevated and often strongly convex, usually without constricted base, 0.8–2(–2.5) mm diam.; thalline margin poorly developed, non-prominent, entire to slightly undulating, white to creamish white; hymenial disc dark brown, covered with a thin layer of whitish pruina giving a white-grey tinge to the apothecial disc, sometimes with cracks. Excipulum thin, almost inconspicuous, brownish, c. 6–10 μm. Hymenium clear, hyaline to pale fawn, 85–115 μm tall, I+ blue turning orange-reddish in parts, KI+ pale blue turning orange in parts; epihymenium pale brown, c. 22–27 μm thick, I+ blue turning orange-reddish in parts, KI+ pale blue. Paraphysoids sparsely branched-anastomosing but richly branched in the epihymenium, 2.5–3 μm thick, not or slightly enlarged to 4 μm and often pale brownish in the epihymenium. Hypothecium dark brown to black, extending to the substratum, up to 1 mm thick, I−, K− or slightly olivaceous. Asci (4–)8-spored, clavate, 65–95 × 15–18 μm, with a tiny ocular chamber; endoascus KI+ blue with a KI+ blue apical ring. Ascospores fusiform, straight to slightly curved, 3-septate, hyaline, (19–)22.5–27(–29) × (4–)4.5–5.5(–6) μm (n = 34); gelatinous sheath not seen.
Pycnidia immersed in the thallus, visible as dark brown dots, surrounded by a thin whitish to cream margin, level with the thallus surface, c. 90–180 μm diam.; spherical in cross-section; wall dark brown; conidia hyaline, filiform, slightly curved to sickle-shaped, 10–16 × 1–1.4 μm.
Chemistry
Thallus C−, I−, K−, PD−, UV−. TLC (solvent G): cf. roccellic acid and an unidentified fatty acid of R f 27 (all specimens tested).
Etymology
The epithet is named after Bruno Mies for his important contribution to the lichen flora of Cape Verde, where the new species was collected, and for having collected the new species first.
Distribution and ecology
So far known only from the islands of São Nicolau and São Vicente in Cape Verde, where it inhabits sheltered volcanic rocks in rather open conditions at relatively high elevations (e.g. near the summit of Monte Verde on São Vicente).
Discussion
The new species is most similar to Syncesia sulphurea (Vainio) Tehler, which differs by having a thallus I+ dark blue in patches, a non-tomentose hymenial disc, a shorter hymenium (55–80 μm) and a chemistry notably with protocetraric acid (Tehler Reference Tehler1997). Syncesia leprobola Tehler differs by the verrucose, nearly isidiate thallus, synascomata and the production of gyrophoric and protocetraric acids in addition to roccellic acid. Syncesia myrticola (Fée) Tehler, widespread in Europe and Macaronesia, can grow both on bark and rocks. It differs from the new species notably by the much longer ascospores (35–44 μm) and the production of protocetraric acid.
Additional specimens examined
Cape Verde: São Vicente: E of Mindelo, Monte Verde, 16°52ʹ22ʺN, 24°55ʹ58ʺW, c. 670 m elev., sheltered volcanic rocks, 2011, D. Ertz 17192 (BR); ibid., Monte Verde along road on the E side of the mountain, 16°52.570ʹN 24°55.680ʹW, 550 m elev., 2010, A. Tehler 10039 (S-F206151). São Nicolau: Punta Espechim, W Ribeira Funda, N-Küste, 16°40ʹN, 24°20ʹW, 280 m elev., N. expos., 1988, B. Mies CV-4296 [divided into five envelopes: 940,2; 940,3; 940,4; 940,5; 940,6] (S-F160224, S-F160225, S-F160226, S-F160227, S-F160228).
New combinations, a new name and emended description of Ingaderia
Ingaderia Darb. emend.
Berichte der Deutschen Botanischen Gesellschaft 15, 6 (1897); type: Ingaderia pulcherrima Darb.
Syn. nov.: Llimonaea Egea & Torrente, in Torrente & Egea, Nova Hedwigia 52, 239 (1991); type: Llimonaea occulta Egea & Torrente.
Syn. nov.: Paraingaderia Ertz & Tehler, Fungal Diversity 49, 56 (2011); type: Paraingaderia placodioidea Ertz & Tehler.
Thallus saxicolous, rarely corticolous, lichenized, crustose, placodioid with subfruticose outgrowths or fruticose, pale creamish or white, sometimes pinkish when fresh or greyish brown, matt, sometimes sorediate, usually rich in crystals notably of calcium oxalate, ecorticate or corticate; cortex when present of ‘branched type’ or of ‘periclinal plectenchyma’ (as defined by Aptroot & Schumm (Reference Aptroot and Schumm2011: p. 7)). Photobiont trentepohlioid.
Ascomata often numerous, elongate to lirellate, rarely punctiform or roundish, immersed or elevated above the thallus, often with a thallus cover, unbranched to densely branched, straight or flexuose; hymenial disc remaining slit-like or exposed, epruinose or pruinose. Excipulum thick, black, c. 10–100(–150) μm thick laterally, K+ becoming slightly darker or olivaceous, merged with the hypothecium below. Hymenium clear, colourless to pale fawn, 75–140 μm tall, usually hemiamyloid; epihymenium hyaline or brown, rarely with an olivaceous tinge. Paraphysoids branched and anastomosing, 1–2.5 μm thick, often slightly enlarged at the apices. Hypothecium dark brown to black, extending to medulla or substratum, 25–400 μm thick. Asci (4–)8-spored, narrowly clavate, not thickened near apex, 50–110 × 10–22 μm, with a tiny ocular chamber; endoascus KI+ blue usually with an apical KI+ blue ring. Ascospores obtusely fusiform or fusiform, sometimes with the upper half wider, (1–)3–7(–14)-septate, thick-walled, often becoming dark brown at an early stage or when old, 15–50 × 4–8(–9) μm, often with a gelatinous sheath c. 0.5–2 μm.
Pycnidia immersed in the thallus, visible as dark brown to black dots, level with or slightly elevated above the thallus surface, c. 90–190 μm diam.; ±spherical in cross-section; wall dark brown; conidia hyaline, filiform, slightly curved to sickle-shaped, 12–24 × 0.7–1 μm.
Chemistry
Often with erythrin, gyrophoric acid and/or lecanoric acid, rarely with psoromic acid, as major secondary metabolites.
Ingaderia flexuosa (Egea, Torrente & Mies) Ertz & Tehler comb. nov. & emend.
MycoBank No.: MB 846085
Llimonaea flexuosa Egea et al., Mycotaxon 53, 63 (1995) [Basionym]; type: Cape Verde Islands, Boa Vista, Mt Gude/Passarao, ombrophobous, shady overhangs, above tuffets and soil, 250 m, N. expos., 9 November 1987, B. Mies 616c, CV-3520 (MUB or BCN—holotype, lost?; FR—former isotype designated here as lectotype! MBT 10010350; F—isotype lost?).
(Fig. 6)

Fig. 6. Ingaderia flexuosa (A, C & G, Mies 616c (isotype FR); B & H, Ertz 17243; D–F, Ertz 13880; I, Mies 835d1). A–D, thallus and ascomata. E, section through an ascoma in water. F–I, ascospores in water. Scales: A–D = 1 mm; E = 100 μm; F–I = 10 μm. In colour online.
Thallus crustose, pale creamish or whitish, continuous to rimose-areolate, flat or bullate, smooth to slightly farinose-rugulose, matt; areoles 0.3–0.6(–1) mm diam., in section c. 0.1–0.7 mm thick; ecorticate or with a poorly developed upper cortex c. 25–30 μm thick, of ‘branched type’ (as defined by Aptroot & Schumm (Reference Aptroot and Schumm2011: p. 7)); rich in crystals (polarized light!), some dissolving in KOH and others of calcium oxalate (H2SO4!). Photobiont trentepohlioid; cells rounded to elongate, 10–20(–25) × 8–15 μm.
Ascomata lirelliform, black, first immersed in the thallus, becoming quickly elevated to sessile, often with a thallus cover c. 55–65 μm thick in the lower third to half part, rarely up to the slit-like opening, simple or rarely with 1(–2) short branch(es), straight or flexuose, (0.3–)0.4–4 × 0.2–0.6 mm; hymenial disc remaining slit-like, epruinose. Excipulum thick, black, 60–100(–150) μm thick laterally, K+ becoming slightly darker-olivaceous, merged with the hypothecium below. Hymenium clear, hyaline, 100–140 μm tall, I+ red, KI+ pale blue; epihymenium hyaline, I+ red, KI+ pale blue. Paraphysoids richly branched-anastomosing, 2 μm thick, not or slightly enlarged to 3 μm at the hyaline apex. Hypothecium dark brown to black, extending to medulla or substratum, 200–400 μm thick. Asci (6–)8-spored, narrowly clavate, not thickened near apex, 85–110 × 15–17 μm, with a tiny ocular chamber; endoascus KI+ blue with an apical KI+ blue ring. Ascospores fusiform, sometimes with the upper half wider, 3–7(–8)-septate, dark brown from an early stage, with a distinct dark brown verruculose ornamentation on the wall, K+ becoming slightly darker, (22–)23.5–29(–32) × (4–)4.5–5.5(–6) μm (n = 40), with a gelatinous sheath c. 1–2 μm.
Pycnidia immersed in the thallus, visible as dark brown to black dots, level with or slightly elevated above the thallus surface, 100–150 μm diam.; ±spherical in cross-section; wall dark brown; conidia hyaline, filiform, slightly curved to sickle-shaped, 12–15(–20) × 1 μm.
Chemistry
Thallus C+ red, K−, UV−. TLC (solvents EA and G): gyrophoric acid (major), often with a trace of lecanoric acid (specimens tested: Ertz 13880, 17228, 17239, 17243, 17273, Mies 835d and lectotype (FR)).
Distribution and ecology
So far known from the islands of Boa Vista, Santiago and São Vicente in Cape Verde and from the island of El Hierro in the Canary Islands, where it inhabits volcanic rock in arid and open conditions near the sea.
Discussion
The holotype of Ingaderia (Llimonaea) flexuosa is missing in MUB and BCN. No specimen could be found by the curator under the name Llimonaea flexuosa, nor under the names ‘Opegrapha undulata Stirton’ (a name under which the specimen Mies 616c (CV-3520) was listed in the thesis of Mies (Reference Mies1989: p. 162), before it was selected as the holotype for the description of Llimonaea flexuosa) or ‘Opegrapha 3’ (an older working name; B. Mies, personal communication). In the original description of L. flexuosa, two isotypes (‘herb. Mies, herb. Lumbsch’) were listed, as well as a paratype (‘Mies 835/836e, CV-4004 (M)’). Fortunately, the isotype from ‘herb. Mies’ was found in FR and the paratype in M, but no isotype could be found in F where hb. Lumbsch is now hosted. The information on the label of the paratype specimen in M slightly differs from the original publication: ‘W of Mt Graciosa’ and ‘Mies 835/836e’ are indicated in the original publication, while ‘N des Mt Graciosa’ and ‘Mies 835d1’ are written on the printed label of the specimen (and ‘835/836d1’ is also written with a pencil on the envelope). However, both have the second collecting number (CV-4004).
The loss of the holotype of Ingaderia flexuosa is problematic because the original description appears to be based on material from two different species. Indeed, the chemistry is described as ‘erythrin and lecanoric/gyrophoric acids detected by TLC’, while we could detect only gyrophoric and lecanoric acids in our specimens. Moreover, the ascomata are described as ‘at first dendroid and immersed, later lirelliform and elevated. Lirellae 1–5 × 0.2–0.7 mm, flexuous, simple or slightly branched’, while we have not observed dendroid lirellae in our specimens. Among the figures of the holotype (Egea et al. Reference Egea, Torrente and Mies1995), fig. 1 shows lirellae that are mostly unbranched-flexuose, elevated and black. These lirellae are similar to those of the isotype in FR (designated here as lectotype) and to our specimens of I. flexuosa, but fig. 4 ‘detail of young ascomata’, though of poor quality, shows lirellae that are richly branched-dendroid, narrow and mostly immersed in the thallus. These latter, dendroid lirellae are somewhat reminiscent of the species described here under the name Ingaderia dendritica, a species that produces erythrin but not lecanoric/gyrophoric acids. Therefore, it is highly probable that the original description of I. flexuosa is based on material of two different species, I. flexuosa s. str. (as accepted here) and I. dendritica, and that the holotype is a mix of these two species. This might explain why the chemistry was described as including erythrin and lecanoric/gyrophoric acids. We decided to consider the isotype in FR as the reference for I. flexuosa (therefore designate that specimen as lectotype) and to describe the second species as I. dendritica. The species of the lectotype (FR) is also the one that appears to constitute most of the holotype of I. flexuosa (see fig. 1 in Egea et al. (Reference Egea, Torrente and Mies1995)) and that better fits the original description in the mature lirellae that are prominent, mostly simple and more robust (0.2–0.7 mm wide, while those of I. dendritica never become wider than 0.4 mm). Since the holotype probably represents a mix of two different species, an emended description of L. flexuosa is provided above using the lectotype (FR) and our recent material.
The lirellae of I. flexuosa as accepted here are quite variable regarding the thallus margin, being sometimes almost devoid of, or almost entirely covered laterally by, a thallus cover. This variability can sometimes be observed within the same thallus (e.g. in the paratype).
The specimen Ertz 13880 was already reported as Llimonaea flexuosa from the Canary Islands (island of El Hierro) by van den Boom & Ertz (Reference van den Boom and Ertz2012).
Additional specimens examined
Cape Verde: Santiago: N des Mt Graciosa, N von Tarrafal, auf Felsblöcken, 200 m elev., N. expos., 1988, Mies 835d1, CV-4004 (M—paratype). São Vicente: S of the road between Baia das Gates and Calhau, NE of Monte Verde, 16°52ʹ29ʺN, 24°54ʹ46ʺW, 200 m elev., on vertical NE-facing coastal volcanic outcrops, on rock, 2011, Ertz 17228, 17239, 17243 (BR); ibid., NW of Calhau, northern slope of Goa Baixo, 16°51ʹ27ʺN, 24°52ʹ42ʺW, c. 120 m elev., strongly sloping and vertical coastal volcanic outcrop, on rock, 2011, Ertz 17273 (BR).—Spain: Canary Islands: El Hierro, Guarazoca, c. 500 m NE of mirador de la Peña, NE-facing rocky outcrop, 27°48ʹ29ʺN, 17°58ʹ46ʺW, 630 m elev., overhang of a small rocky outcrop, 2009, Ertz 13880 (BR).
Ingaderia occulta (Egea & Torrente) Ertz comb. nov.
MycoBank No.: MB 846086
Llimonaea occulta Egea & Torrente, in Torrente & Egea, Nova Hedwigia 52, 239 (1991) [Basionym]; type: Portugal, Estremadura, Sintra, Cabo Roca, 20–140 m elev., 19 February 1987, J. M. Egea (MUB 13619—holotype).
Ingaderia placodioidea (Ertz & Tehler) Ertz & Tehler comb. nov.
MycoBank No.: MB 846087
Paraingaderia placodioidea Ertz & Tehler, Fungal Diversity 49, 56 (2011) [Basionym]; type: Yemen, Socotra Island, Sefflah, the ridge just S of the village on S coast at the E most part of the island, 400–600 m elev., 12°30ʹ43.4ʺN, 54°26ʹ02.2ʺE, on limestone, 24 March 2008, A. Tehler 9344 (S—holotype!; BR—isotype!).
Ingaderia sorediata (Sparrius, P. James & M. A. Allen) Ertz comb. nov.
MycoBank No.: MB 846088
Sclerophytonomyces circumscriptus var. sorediatus Sparrius et al. [as ‘Sclerophytomyces’], Lichenologist 37, 285 (2005) [Basionym].—Peterjamesia sorediata (Sparrius et al.) D. Hawksw., Lichenologist 38, 189 (2006).—Roccellographa sorediata (Sparrius et al.) Coppins & Fryday, in Fryday & Coppins, Lichenologist 44, 734 (2012).—Fulvophyton sorediatum (Sparrius et al.) van den Boom [as ‘sorediata’], in van den Boom & Giralt, Sydowia 64, 152 (2012) nom. inval. (Art. 41.5).—Fulvophyton sorediatum (Sparrius et al.) Tehler & van den Boom, in Tehler, Lichenologist 49, 173 (2017); type: Spain, Canary Islands, La Palma, Lomo Machín, Barlovento, 250 m elev., 21 September 1979, C. Hernández Padron (E 197301—holotype; TFC 849, BM—isotypes).
Discussion
Sclerophytonomyces circumscriptus var. sorediatus (Sparrius et al. Reference Sparrius, James and Allen2005) was erected to species level by Hawksworth (Reference Hawksworth2006) as Peterjamesia sorediata, because he considered it to be genotypically distinct from the non-sorediate taxon (= P. circumscripta (Taylor) D. Hawksw.) since it grows with it and does not intergrade with it. In a publication on the phylogeny of the Arthoniales, Ertz & Tehler (Reference Ertz and Tehler2011) sequenced P. circumscripta and found that the genus Peterjamesia D. Hawksw. should be included under the earlier generic name Roccellographa J. Steiner. As a consequence, the type species of the former genus (P. circumscripta) was transferred into Roccellographa. Subsequently, P. sorediata was transferred to Roccellographa by Fryday & Coppins (Reference Fryday and Coppins2012), despite the species not being sequenced. However, van den Boom & Giralt (Reference van den Boom and Giralt2012) simultaneously transferred it to Fulvophyton because they found a fertile specimen having notably roundish to shortly lirellate ascomata immersed in the thallus, a brownish excipulum, a hyaline hypothecium and (4–)6–8-septate, hyaline ascospores, 20–35 × 5–7 μm, with a distinct gelatinous sheath. This latter combination was invalid and was eventually validated in Tehler (Reference Tehler2017). The sequencing of several specimens during the present study revealed that the species was related to neither Roccellographa circumscripta nor even to the family Roccellographaceae (where the genera Roccellographa and Fulvophyton belong) but to the family Opegraphaceae. Following the phylogenetic results (Fig. 2), F. sorediatum is transferred to the genus Ingaderia. The generic concept of Ingaderia is enlarged by the inclusion of a species producing psoromic acid. Specimen Ertz 16878 has many lirellae, but only a small number of ascospores were observed that fit the description of van den Boom & Giralt (Reference van den Boom and Giralt2012): hyaline, 7-septate, 25–26 × 5 μm. However, in our specimen, the wall of the ascospores becomes brown when overmature and a thick dark brown hypothecium is present.
Sequenced specimens (all sterile, except Ertz 16878 with many lirellae)
France: Brittany: Finistère Dept., Camaret-sur-Mer, Pointe de Pen Hir, 35 m elev., affleurement rocheux siliceux de bord de mer, entouré de landes, sur rocher abrité, 2012, D. Ertz 17469 (BR). Corsica: Marchese (Cargèse), Punta d'Omigna, entre le Golfe de Chiuni et le Golfe de Peru, c. 30 m elev., sur rocher, 2011, D. Ertz 16412 (BR).—Portugal: Azores: Pico, between Sao Miguel Arcanjo (Sao Roque do Pico) and Prainha de Cima, c. 90 m elev., on rock, 2011, D. Ertz 16690 (BR); Terceira, NE of Serreta, Ponta do Queimado, c. 30 m elev., volcanic rock near the sea, 2011, D. Ertz 16878 (BR). Algarve Prov.: SW of Aljezur, c. 40 m elev., on rock, 2011, D. Ertz 16917 (BR). Estremadura Prov.: Sintra, Cabo da Roca, c. 50 m elev., siliceous outcrops, 2011, D. Ertz 17049 (BR).
Ingaderia vandenboomii Ertz nom. nov.
MycoBank No.: MB 846089
Llimonaea sorediata van den Boom et al., in van den Boom & Brand, Lichenologist 39, 310 (2007) [replaced synonym] non Ingaderia sorediata (Sparrius et al.) Ertz, in Ertz & Tehler, Lichenologist: this publication; type: Portugal, Estremadura, E of Nazaré, São Bartolomeu, on top of small rocky hill, on E-exposed overhanging outcrops, 9°03.7ʹW, 39°35.5ʹN, 150 m elev., 15 July 2001, P. & B. van den Boom 27645 (LG—holotype; hb. v.d. Boom—isotype).
Discussion
Since Fulvophyton sorediatum and Llimonaea sorediata need to be combined in Ingaderia (see Discussion below), and since both species have the same epithet, a new name has to be introduced for one of the two species. We decided to introduce a new name for the most recently described epithet (thus for L. sorediata) and to dedicate the new name to Pieter van den Boom for his important contribution to lichenology and the discovery and description of this species.
Discussion
Ingaderia is a genus described by Darbishire in 1897 for I. pulcherrima Darb., a fruticose lichen endemic to the coasts of Chile (Darbishire Reference Darbishire1897). The ascomata were described (and illustrated) as simple to ramified and elongate lirellae lacking a thalline margin, with a brownish black excipulum and hypothecium, thus similar to species of Opegrapha. Tehler (Reference Tehler1990) provided a detailed description of I. pulcherrima, characterizing the thallus as fruticose, pendent, corticate, with complanate or roundish branches, often with apically black papillae from which pycnidia often develop, with filiform and curved conidia and producing erythrin, lecanoric acid and an unidentified blue fluorescent substance. However, as I. pulcherrima is often infected by parasites, Tehler (Reference Tehler1990) concluded that the ascomata originally described as the mycobiont by Darbishire (Reference Darbishire1897) refer to those of a lichenicolous species, and as a consequence the ascomata of I. pulcherrima were considered as unknown. Nevertheless, I. pulcherrima has been placed in the family Opegraphaceae in a molecular phylogenetic study (Ertz & Tehler Reference Ertz and Tehler2011) and our new phylogenetic data (Fig. 2) place Ingaderia close to taxa producing similar ascomata (i.e. lirellate with a thick black excipulum and hypothecium). Therefore, the ascomata described originally by Darbishire (Reference Darbishire1897) for Ingaderia are very likely to be those of the mycobiont. Moreover, Tehler (Reference Tehler1990: p. 2477) provided a detailed description of this type of ascomata in I. pulcherrima and described the ascospores as ‘7-septate, thick-walled, not constricted at septa, narrowly ellipsoidal, straight, hyaline, 20–25 × 4–5 μm’. The ascospores are thus also similar to those observed in most of the taxa belonging to the same lineage as Ingaderia pulcherrima (e.g. Llimonaea flexuosa, L. occulta, Fulvophyton sorediatum), though the ascospores often become dark brown.
Ascospores that are dark brown at maturity in the inner part of the cell walls are a rare feature in the Arthoniales, but not uncommon in the Opegraphaceae. In the lineage Sparria caboverdensis to Llimonaea occulta (Fig. 2), it is shared by species of Llimonaea, Paraingaderia placodioidea, Sparria caboverdensis, S. cerebriformis, Schizopelte californica Th. Fr. and S. crustosa Ertz & Tehler. However, the colour of the ascospores might vary with maturity even within a species. In L. flexuosa, ascospores are dark brown in an early stage or at maturity in most specimens, but we mainly observed hyaline mature ascospores in specimen Ertz 17243 (Fig. 6H). In our sequenced specimens of Llimonaea occulta, ascospores are still hyaline at maturity and become brown only when old.
With the phylogenetic placement of Llimonaea flexuosa outside the strongly supported lineage Fulvophyton sorediatum-Llimonaea occulta (Fig. 2), generic delimitation becomes problematic. Based on the phylogeny, there are four possibilities for the generic delimitation of the lineage Llimonaea flexuosa-L. occulta.
1) Five genera: the genus Llimonaea (including L. occulta and L. sorediata) / the monotypic genus Ingaderia / the monotypic genus Paraingaderia / a new genus for Fulvophyton sorediatum / a new genus for Llimonaea flexuosa.
2) Four genera: same as for the five genera, but with Paraingaderia included in Llimonaea.
3) Three genera: Fulvophyton sorediatum and Paraingaderia are included in Llimonaea together with L. occulta and L. sorediata / the monotypic genus Ingaderia / a new genus for Llimonaea flexuosa.
4) One genus: all the taxa are included in the genus Ingaderia, the oldest generic name available.
There are few morphological characters that can be used to give generic rank to subgroups within the lineage Llimonaea flexuosa-L. occulta. All taxa share a developed dark brown to black excipulum and hypothecium, ascospores that are elongate-fusiform, thick-walled, transversally septate and medium-sized (c. 15–50 × 4–8 μm) and conidia that are filiform-curved (not seen in L. sorediata and F. sorediatum). The genera Ingaderia and Paraingaderia have fruticose and subfruticose thallus growth forms, respectively, contrasting with the crustose thallus of the other lichens. However, genera with a diversity in thallus growth form, ranging from crustose to fruticose thalli, are already known in the Arthoniales, viz. the genera Dendrographa, Pentagenella and Roccellina (Tehler & Irestedt Reference Tehler and Irestedt2007; Ertz & Tehler Reference Ertz and Tehler2011; Perlmutter et al. Reference Perlmutter, Rivas Plata, LaGreca, Aptroot, Lücking, Tehler and Ertz2020). A thalline margin laterally covering the black excipulum is absent in Ingaderia but characterizes the genus Llimonaea, while Fulvophyton sorediatum and Paraingaderia placodioidea have ascomata immersed in the thallus (and thus can be considered as having a lateral thalline margin). However, in Llimonaea flexuosa this character is highly variable even within a specimen, with a thalline margin absent or almost entirely covering the excipulum. In the Graphidaceae, a high diversity in thallus cover is observed within the lirellate genus Graphis, with species lacking a thalline margin to species having a complete and thick thalline margin (Lücking et al. Reference Lücking, Archer and Aptroot2009). Erythrin and lecanoric acid are produced by all taxa of the lineage Llimonaea flexuosa-L.occulta, except L. flexuosa (gyrophoric acid) and Fulvophyton sorediatum (psoromic acid), but the chemistry is often variable within genera. As a consequence, a split into five, four or three genera would lead to poorly characterized, potentially mainly monotypic genera. Accepting Llimonaea, even by including Paraingaderia and possibly also Fulvophyton sorediatum, would make Llimonaea paraphyletic, while a new genus for L. flexuosa is difficult to justify. We instead suggest including all taxa of the lineage Llimonaea flexuosa-L.occulta in Ingaderia. This results in a phylogenetically distinct and enlarged concept of Ingaderia that, however, is rather difficult to characterize morphologically. The sequencing of more crustose lirellate Arthoniales, in particular from South America, will be essential to make further progress in our understanding of the evolution in this subgroup of the Opegraphaceae.
Acknowledgements
Christian Printzen and Andreas Beck are thanked for the loan of the isotype of Llimonaea flexuosa from FR and the paratype of L. flexuosa from M, respectively. Roser Guardia and Thorsten Lumbsch are thanked for searching and confirming that no specimen of Llimonaea flexuosa was present in MUB and BCN, and F respectively. We wish to warmly thank Lynn Delgat and Wim Baert (Meise Botanic Garden) for their help with the molecular work.
Author ORCIDs
Damien Ertz, 0000-0001-8746-3187; Anders Tehler, 0000-0002-4171-5497.
Competing Interests
The authors declare none.










