Despite n-3 long-chain PUFA (LCPUFA) have been long recognised as being important for brain development and function, little is known on their specific effects on neuron activity in relation to behaviour. n-3 LCPUFA play important roles in neural growth, development of synaptic processing of neural cell interaction and expression of genes that regulate cell differentiation and growth(Reference Uauy and Dangour1). Essential fatty acid metabolism can influence many aspects of brain development, including neuronal migration, axonal and dendritic growth and the creation, remodelling and pruning of synaptic connections(Reference Guesnet and Alessandri2, Reference Robson, Dyall and Sidloff3). Animal studies have shown that both neural integrity and function can be permanently disrupted by deficits of n-6 and n-3 fatty acids during fetal and neonatal development(Reference Yamamoto, Hashimoto and Takemoto4, Reference Bourre, Francois and Youyou5). While both n-6 and n-3 fatty acids are required, the n-3 fatty acids, such as DHA (22 : 6n-3), appear to play a special role in highly active sites such as synapses and photoreceptors and deficiencies have particularly been linked to visual and cognitive deficits(Reference Neuringer, Connor and Lin6, Reference Neuringer, Reisbeck and Janowsky7).
In marine fish, n-3 LCPUFA are essential and play very important physiological roles(Reference Izquierdo, Watanabe, Takeuchi, Takeda and Watanabe8, Reference Izquierdo9). Specifically, DHA and EPA (20 : 5n-3) acids must be supplied in the diet and function as critical structural and physiological components of the cell membranes of most tissues that are necessary for fish growth, welfare, survival and development(Reference Watanabe and Kiron10, Reference Izquierdo, Socorro and Arantzamendi11). In particular, DHA has been found to be required for the normal development of the nervous system and sensory organs, such that the larval brain and eye fatty acid compositions reflect the diet(Reference Masuda, Takeuchi and Tsukamoto12, Reference Benítez-Santana, Masuda and Valencia13). Moreover, DHA deficiency impairs vision in juvenile herring (Clupea harengus)(Reference Bell, Batty and Dick14). Therefore, dietary fatty acid contents could potentially affect behaviour, stress reactions or pain and comfort, despite the lack of studies on this subject in fish. Recently, dietary fatty acids have been found to affect escape and avoidance behaviour in fish larvae after a sound or visual stimulus(Reference Benítez-Santana, Masuda and Valencia13, Reference Masuda, Shoji and Aoyama15). Fish can elude predatory attacks by producing a stereotyped escape behaviour, which is characterised by a rapid and powerful unilateral bending of the body and caudal fin that involves most of its somatic musculature(Reference Korn and Faber16). This behaviour is initiated by the activation of the Mauthner cells (M-cells).
M-cells integrate diverse sensory inputs(Reference Faber, Fetcho and Korn17, Reference Eaton, Lee and Foreman18) and are able to reset swimming rhythms in the course of its initiation of escape behaviours(Reference Svoboda and Fetcho19). In chronic recordings of freely swimming intact fish, the M-cells have been shown to fire an action potential at the initiation of C-start responses(Reference Zottoli20–Reference Weiss, Zottoli and Faber23). Because the axons of each M-cell decussate, the firing of one leads to a contraction of the trunk musculature that is contralateral to the cell soma(Reference Zottoli20, Reference Foreman and Eaton24). To date there is no evidence of an effect of the essential fatty acids on M-cell activity. Therefore, the aim of the present study was to better understand the effect of dietary n-3 LCPUFA on fish escape and avoidance behaviour and neural function. For that purpose the effect of two different lipid sources, with different n-3 LCPUFA content, fed to gilthead seabream (Sparus aurata) during early larval and brain development on the fish reaction to a sonorous stimulus and M-cells activity was investigated. M-cell activity was determined by choline acetytransferase distribution by immuno-labelling as a marker of cholinergic neuron density, since the cholinergic neurotransmission system has been found to be sensitive to dietary n-3 PUFA in rats(Reference Aïd, Vancassel and Poumes-Ballihaut25).
Material and methods
All animal studies complied with the guidelines for animal experimentation of our laboratories and were approved by the institutional review boards.
Experimental conditions
A total of 6600 gilthead seabream larvae of 22 d of age (post-hatch) (5·06 (sd 0·59) mm in standard length) were randomly distributed into six 170 litres fibre glass cylindrical tanks and were fed in triplicate with two experimental diets. Diets differed only in the lipid source to obtain different fatty acid profiles. Lipid sources were fish oil rich in n-3 LCPUFA and soyabean oil rich in linoleic acid (18 : 2n-6). The diets were analysed for crude lipid content and fatty acid composition (Table 1). Microdiets were prepared according to Atalah et al. (Reference Atalah, Hernández-Cruz and Benítez-Santana26). No significant differences were found in the dry lipid content (20·09; P>0·05.). All tanks were supplied with filtered seawater (34 ppm salinity) at 19–20°C, constant aeration (125 ml/min), seawater flow (0·4 litres/min at the beginning to 1·0 litres/min at the end) and artificial light (12 h photoperiod). Feeds (2·5 g/tank per d) were manually supplied fourteen times per d every 45 min during the light period.
Table 1 Fatty acid composition of total lipids of gilthead sea bream larvae fed with fish oil and soyabean oil microdiets (% total determined fatty acids)
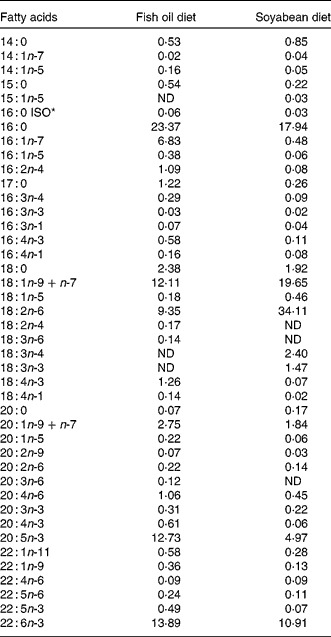
ND, not detected.
* ISO indicates the position of the methyl terminal or subterminal.
Swimming speed behaviour
The swimming speed of the larvae from each tank was determined at 23, 27 and 34 d of age in a 1 litre black wall glass beaker (10 cm diameter) keeping a water depth of 4 cm. Each larva was transferred from the feeding tanks to the glass beaker and then video-recorded using a Sony digital video camera (DCR-TRV27; Sony, Tokyo, Japan). After recording for 30 s, the larva was scared by a sound stimulus to induce a startle response and determine a burst swimming speed. Consistent sound stimuli were produced using a steel nut(Reference Benítez-Santana, Masuda and Valencia13). Sound stimuli were provided three times at 10 s intervals for each larva. Afterwards, larval standard length was measured by a profile projector (Nikon V-12A; Nikon, Tokyo, Japan). This procedure was repeated using ten individuals from each rearing tank. Frame-by-frame video image analysis was conducted to calculate burst swimming speed(Reference Benítez-Santana, Masuda and Valencia13, Reference Masuda, Shoji and Aoyama15). Burst swimming speed was analysed only when the larva showed an obvious startle response. The fastest movement always appeared on the first frames after providing a stimulus as it occurs in younger larvae(Reference Benítez-Santana, Masuda and Valencia13). The speed was presented as a function of the standard length of each individual to avoid larval size interference(Reference Benítez-Santana, Masuda and Valencia13, Reference Masuda, Shoji and Aoyama15).
Biochemical analyses
At the end of the study the larvae were sampled for lipid and fatty acid composition of total lipids. The lipids were extracted by chloroform–methanol mixture(Reference Folch, Lees and Stanley27). Methyl esters of fatty acids were prepared by transesterification with 1 % sulphuric acid and methanol using heneicosanoic acid (21: 0; 10 % of total lipids) as an internal standard(Reference Christie28). The fatty acid methyl esters obtained were separated by a GC (Shimadzu GC-14a, Kyoto, Japan) run using the operating conditions described previously(Reference Izquierdo, Watanabe, Takeuchi, Takeda and Watanabe29), quantified by flame ionisation detection and identified by comparison with well-characterised external standards.
Immunofluorescence study
A total of thirty larvae per tank (n 90) were collected and fixed in 10 % buffered formalin at the end of the experiment. Each larva head was mounted in a gelatin block in a horizontal orientation to obtain better visualisation of neuronal structures. Gelatin-embedded larva head blocks were cryoprotected in 30 % sucrose and serially cut on a Slee Mainz cryostat at 10 μm. Each gelatin section for immunofluorescence slides was collected on gelatin-coated slides. The antibody was tested to determine the optimal working concentration and quality of the signal and seabream larvae were processed for the demonstration of immunoreactivity. The slides were covered with 5 % rabbit serum and 0·2 % Triton in PBS for 1 h. Incubations with anti-choline acetyltransferase primary antibody (1:250; Millipore, Billerica, MA, USA) were carried out for 48 h at 4°C in a 0·1 m (PBS) solution with 0·1 % Triton X-100 and 2 % goat serum (Vector). As a secondary antibody we used a anti-goat IgG FITC conjugate (1:250, antibody developed in rabbit affinity-isolated antigen-specific antibody (Sigma, Madrid, Spain) diluted in PBS and applied for 1 h in a dark room at room temperature. A propidium iodide complex at a concentration of 1:104 was applied for 5–10 min at room temperature and was used for nuclei contrast staining. In order to see the M-cell green immunofluorescence, a Zeiss LSM 510 confocal system (Zeiss, Thornwood, NY, USA) was used. In order to prevent fluorescence data from being compromised, in each batch of samples processed all treatments were always included. Negative controls were run by replacing each primary antibody by PBS to test for the specificity of an antibody involved.
Statistical treatment of data
Data were statistically analysed with the SPSS software (SPSS 11.5 for Windows; SPSS, Inc., Chicago, IL, USA). The values are reported as means and standard deviation. The normality of the variable distribution was verified using Levene's test, not requiring any transformation. The values of fatty acid levels were expressed as means and standard deviation, and Student's t test was employed to compare the results of fatty acids and behavioural studies. Significance was accepted at P < 0·05.
Results
Under the stimulus challenges conducted during the study, the number of larvae (twenty larvae/diet per challenge) that reacted to the sound stimulus was related to larval age and diet. Thus, at 23 d of age a low percentage of larvae reacted to the stimulus, whereas at 27 d this number significantly (P < 0·03) increased (Fig. 1 (a)). At 32 d, a similar proportion of larvae reacted when they were fed fish oil, whereas the percentage of reacting larvae was lower in those fed soyabean oil (P < 0·05; Fig. 1 (a)).
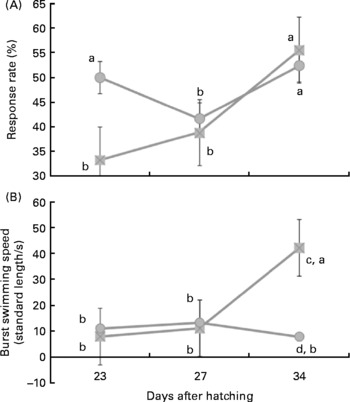
Fig. 1 Larval reaction after the sound stimuli along larval development. (A) Number (%) of reacting sea bream larvae for each experimental group after the sound stimuli fed diets containing fish oil (![]() ) and soyabean oil (
) and soyabean oil (![]() ). (B) Burst swimming speed (standard length/s) in sea bream larvae fed microdiets enriched with different types of lipids: fish oil (
). (B) Burst swimming speed (standard length/s) in sea bream larvae fed microdiets enriched with different types of lipids: fish oil (![]() ) and soyabean oil (
) and soyabean oil (![]() ). a,b Mean values with unlike letters were significantly different between animals of same treatment (n 30, P < 0·05). c,d Mean values with unlike letters were significantly different between animals of different treatment (n 30, P < 0·05).
). a,b Mean values with unlike letters were significantly different between animals of same treatment (n 30, P < 0·05). c,d Mean values with unlike letters were significantly different between animals of different treatment (n 30, P < 0·05).
In larvae fed fish oil, burst swimming speed after sound stimuli increased with age being highest in larvae of 34 d (P < 0·03; Fig. 1 (b)). However, in larvae fed soyabean oil burst swimming speed only increased at 27 d in comparison with 23 d and it was even reduced in 34-d-old larvae when they were fed soyabean oil (Fig. 1 (b)). Therefore, at day 34 burst swimming speed after sound stimulus of larvae fed fish oil was significantly higher (P < 0·02) than that of fish fed soybean oil (Fig. 1 (b)).
Confocal microscopy analysis showed a greater acetylcholine immunopositive response (green fluorescence) in larvae fed the fish oil microdiet than in those fed the soyabean oil microdiet (P < 0·03; Fig. 2). Fluorescence quantification showed a significantly higher immunoposititve response in larvae fed fish oil than in larvae fed the soyabean oil microdiet (Fig. 3).
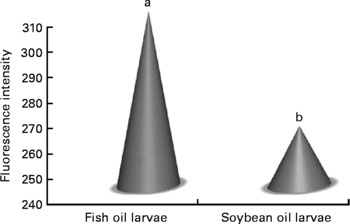
Fig. 2 Quantification of green fluorescent intensity by confocol microscopy according to the immunopositive response of Mauthner cells in sea bream larvae fed microdiets with fish oil and soyabean oil. a,b Mean values were significantly different between animals of different treatment (n 30, P < 0·05).
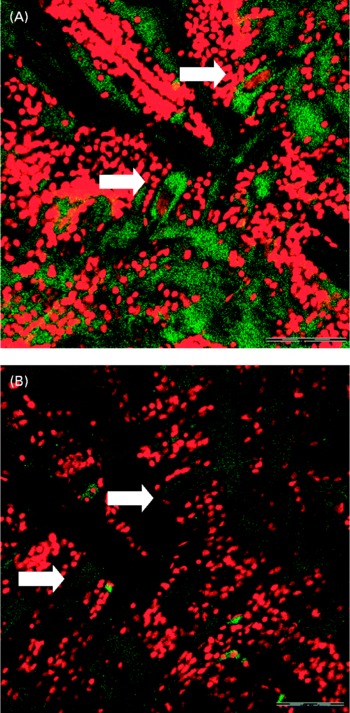
Fig. 3 Confocol microscopy image of the acetylcholine immunopositive response of Mauthner cells longitudinal sections from larvae fed with (A) fish oil microdiet and (B) soyabean oil microdiet. Scale bar 10 μm.
The larvae fed the fish oil diet showed the highest DHA and EPA (P < 0·03) content in the whole body (Table 2). The fatty acid composition of larval fish (Table 2) at 36 d after hatching showed that larvae fed with soyabean oil microdiets lost n-3 LCPUFA content (both EPA and DHA in the same proportion) in comparison with larvae fed with a fish oil microdiet in which the content of DHA and EPA (P < 0·004) is higher in whole body compared with fish fed soyabean oil. Larvae fed the fish oil microdiet showed a reduction in monoenoics and unsaturated fatty acids, while showing the highest content of n-3 fatty acids. Larvae fed with soyabean oil microdiets were rich in fatty acids of the n-6 series, particularly 18 : 2n-6 showed a significant reduction (P < 0·04) compared with levels found in fish fed the fish oil diet. Soyabean oil larvae showed a higher content of monoenoics and SFA, which may reduce membrane fluidity. These fatty acid incorporation results demonstrated good assimilation of the diet by the larvae.
Table 2 Fatty acids content (% total determined fatty acids, n 3) of 35 d old sea bream larvae fed with fish oil microdiet (fish oil larvae) and soyabean oil microdiet (soyabean larvae)
(Mean values and standard deviations)
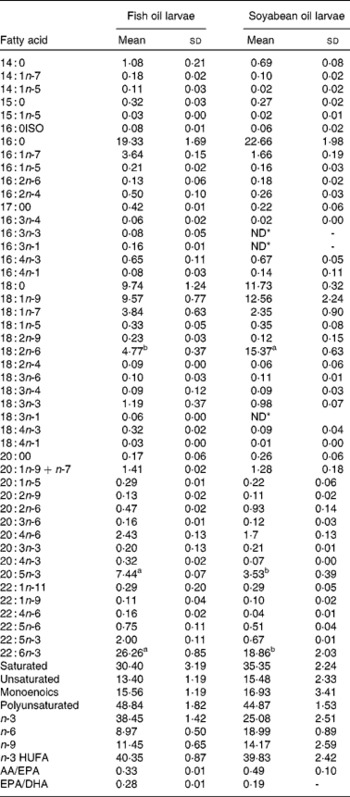
ND, not detected.
a,b Mean values within a row with unlike superscript letters were significantly different as determined by Student's t test honestly significant difference (n 3, P < 0·05).
* ND ≤ 0·005
Discussion
In the present study, the reduction in dietary n-3 PUFA caused a significant reduction in the contents of these fatty acids in the fish. In gilthead seabream, brain fatty acid composition is also modified by the n-3 LCPUFA content of the diet(Reference Benítez-Santana, Masuda and Valencia13), and therefore, being necessary for the normal development of nervous system and sensory organs, these fatty acids could affect physiological functions in the brain. In fact, a diet unbalanced in n-3 PUFA may cause changes in cell permeability and synaptic membrane fluidity(Reference Yehuda, Rabinovitz and Mostofsky30), or modifications in the number and affinity of receptors, in the function of ion channels and on the activity of neurotransmitters(Reference Jump31). Thus, alterations in brain fatty acid composition could potentially affect behaviour.
An important behaviour to escape from predation is the startle response, which is initiated by a sudden stimulus and results in rapid reaction. In the present study, the substitution of soyabean oil by fish oil in microdiets for larval gilthead seabream markedly increased n-3 LCPUFA content in fish tissues and affected fish behaviour in terms of the startle response to a sonorous stimulus. Thus, during fish development, a higher but not statistically different number of larvae reacted to the stimulus when they were fed fish oil, rich in n-3 LC-PUFA. Moreover, a n-3 LC-PUFA increase in the diet also led to a faster swimming speed burst in those larvae that reacted to the sonorous stimulus at 32 dph. Therefore, dietary reduction in n-3 LC-PUFA impaired fish larval response to the stressor, reducing the escape behaviour in larvae with a lower content of n-3 LC-PUFA in their body tissues. These results highlight the important role of these fatty acids in response to a sonorous stimulus, in agreement with their importance for sensory organ functioning(Reference Izquierdo32). In contrast with the present study, younger and less developed gilthead seabream burst swimming response to a sound stimulus was not affected by dietary n-3 LCPUFA, whereas they had a high burst swimming speed after a light stimulus(Reference Benítez-Santana, Masuda and Valencia13). This suggests that despite the fact that neural and muscular responses were well developed in those young larvae since they were able to react to a visual stimulus, the reception to the sound stimulus was not sufficiently developed to produce a consistent response according to dietary differences. The mechanoreceptive neuromast cells associated with the lateral line system and the inner ear (auditory nerve) are the major receptors for external vibrational and gravitational stimuli in fish. The lateral line system of teleost fish typically consists of a row of pores along the tail, body and head, leading into an underlying fluid-filled lateral line canal. The neural impulses from these receptors are transmitted along the anterior and posterior lateral line nerves to the octavolateralis area of the medulla(Reference Bleckmann, Bullock and Jorgensen33). In the fish used in the present study the lateral line was better developed, whereas in the former trial, conducted with younger larvae, the lateral line had not started to appear until the end of the experiment (15–20 d). A further development of the sensorial organs in the larvae of the present study would allow a different perception of the stimulus by larvae fed different n-3 LC-PUFA suggesting the importance of these fatty acids for the normal functioning of the lateral line.
The Mauthner neurons are known to receive sensory information not only from the auditory nerve(Reference Faber, Korn and Lin34, Reference Zottoli, Bentley and Prendergast35), but also from the optic tectum(Reference Canfield36), the lateral line mechanosensory system(Reference Zottoli, Danielson, Coombs, Görner and Münz37), the somatosensory channels(Reference Chang, Lin and Faber38) and via the electrosensory system in weakly electric fish(Reference Zottoli, Danielson, Coombs, Görner and Münz37). Thus, a variety of sensory modalities could modulate the relative excitation or inhibition of the M-cells before a startling stimulus driving it beyond threshold levels. Escaping behaviour in fish has been particularly related to M-cells. This pair of reticulospinal neurons initiates fast startle responses in fishes and amphibians and constitutes an important model system in the studies of vertebrate neurons and their control of behaviour(Reference Korn and Faber16). In the present study, faster startle response in fish fed n-3 LCPUFA was also associated with an increased immunopositive neural response, particularly in M-cells, denoting a higher production of acetylcholine. Acetylcholine release in the hippocampus has been found to be reduced under neuronal activation in rats receiving a chronically n-3 PUFA-deficient diet(Reference Aïd, Vancassel and Poumes-Ballihaut25, Reference Kodas, Galineau and Bodard39). n-3 LC-PUFA, and particularly DHA, markedly affect membrane fluidity and functioning and have been found to be important for neurocyte myelination and synapse construction, with both functions being sensitive to nutritional deficiencies(Reference Krigman and Hogan40). Moreover, these fatty acids are nutritional antioxidants that prevent the formation of cerebral lipid peroxides(Reference Choin-Kwon, Park and Lee41), stabilising the oxidant/antioxidant status of the membrane structures in the brain(Reference Sarsilmaz, Songur and Ozyurt42), and protecting them from several neurological and neuropsychiatric disorders(Reference Black, Hoff and Radins43). Most of the DHA accumulation occurs during late pre-natal and early post-natal development, coinciding with the formation of synapses(Reference Green, Glozman and Kamensky44). Similarly, DHA accumulates in the brain and the sensorial organs of the fish during larval development and it is retained in the neural tissues even during periods of starvation(Reference Izquierdo32, Reference Izquierdo, Tandler and Salhí45). Adequate dietary availability of DHA during this period is essential for optimal development and functioning of the central nervous system. Inadequate intake of DHA is thus associated with impaired attention and learning performance as well as modifications in emotional status including elevated behavioural indices of anxiety, aggression and depression(Reference Fedorova and Salem46).
The present study shows the first evidence of the importance of n-3 LCPUFA for the adequate functioning of particular neurons, the M-cells, and, subsequently, for the behavioural response that they modulate to escape from a sound stimulus. Further studies are being conducted to understand the role of these essential fatty acids on neural development and functioning.
Acknowledgements
A doctoral scholarship from the Canarian Government supported the present study. The experiments were designed according to the Animal Welfare Ethics Committee guidelines of Las Palmas de Gran Canaria University. T. B. S. carried out the animal experiment, performed the biochemical and immunofluorescence studies, participated in the interpretation of the results and drafted the manuscript. E. J. C. participated in the design of the study and took part in animal feeding and sampling. M. B., S. T. and M. J. C. participated in the immunofluorescence studies and result interpretation. M. S. I. conceived the study and its design, coordinated the work, and participated in the interpretation of the results and the preparation of the manuscript. All the authors read and approved the final manuscript. There are no potential conflicts of interest.







