Multiple sclerosis (MS) is an autoimmune disease of the central nervous system, with unknown etiology, characterised by chronic inflammation, demyelination and neuronal loss(Reference Vidmar, Maver and Drulović1). Around 2·5 million individuals, worldwide, are affected by this disease(Reference Sowmya and Khader2), although young adults and females are more susceptible(Reference Jamebozorgi, Rostami and Pormasoumi3). Relapsing-remitting MS (RRMS), the most common type of MS, is indicated in, roughly, 85 % of patients(Reference Dolati, Babaloo and Jadidi-Niaragh4).
Contentions in the literature regarding the relationship between some dietary components and MS progression are evident. For example, dietary polyphenols have been reported to mitigate demyelination(Reference Wang, Wang and Yang5), whereas resveratrol – a polyphenol compound found in a variety of foods and beverages – reportedly exacerbated both autoimmune and viral models of MS(Reference Sato, Martinez and Shahid6). Milk proteins and gluten may worsen the clinical manifestations in MS patients(Reference Von Geldern and Mowry7); however, milk consumption more than once per week was found to decrease the risk of developing MS(Reference Halawani, Zeidan and Kareem8). Furthermore, high doses of vitamin C have been shown to worsen MS conditions(Reference Khosravi-Largani, Pourvali-Talatappeh and Rousta9), while some authors have reported that vitamin C promotes oligodendrocytes generation and remyelination(Reference Guo, Suo and Cui10). Indeed, more nutrition-based research is required to clarify these conflicting findings.
Among the most advocated healthy diets, the Mediterranean diet (MeD) has the strongest evidence for improvement in inflammatory status(Reference Paknahad, Sheklabadi and Moravejolahkami11). This diet is characterised by high intake of vegetables, legumes, fruits, whole grains and unsaturated fatty acids (mostly in the form of olive oil), a moderately high intake of fish and low to moderate intake of dairy products, meat and poultry(Reference Moravejolahkami and Chitsaz12). Indeed, previous studies have shown the potential effects of anti-inflammatory diets, such as Mediterranean-style diets, in reducing fatigue severity in MS patients(Reference Moravejolahkami and Chitsaz12–Reference Moravejolahkami, Paknahad and Chitsaz14).
Dietary Inflammatory Index (DII), a literature-based scoring system, is a tool used to classify forty-five pro or anti-inflammatory dietary items into an overall score(Reference van Duijnhoven, Brouwer and van Woudenbergh15). Previous studies have reported that several foods and nutrients used in the DII calculation, such as whole grains, fruits, vegetables, fish, onion and ginger, possess anti-inflammatory effects(Reference Mutthuraj, Vinutha and Gopenath16,Reference Simin, Mitić-Ćulafić and Pavić17) . In contrast, refined grains, red meat, high-fat dairy products and sweats have been routinely related to systemic inflammation(Reference Mahabamunuge, Simione and Horan18). In previous studies, MeD reportedly yielded a strong anti-inflammatory DII score(Reference Mayr, Thomas and Tierney19), and greater MeD adherence has been negatively associated with DII scores(Reference Ruiz-Canela, Zazpe and Shivappa20,Reference Hodge, Bassett and Shivappa21) . On the other hand, some findings suggest that higher DII scores during adolescence might be an important risk factor for MS onset(Reference Abdollahpour, Jakimovski and Shivappa22).
Therefore, given the equivocality present within the literature, we sought to determine the effect of mMeD v. TID, on DII, disease disability and fatigue severity in RRMS patients. We hypothesised that the modified form of MeD (mMeD; mainly by elimination of alcohol-containing foods and beverages) would yield a lower DII score (i.e. greater dietary anti-inflammatory potential) in comparison with the traditional Iranian diet (TID).
Materials & methods
Study design and sample size determination
In this single-centre, two parallel arms, single-blind, randomised clinical trial, 180 RRMS patients were recruited, according to the Extended Disability Status Scale (EDSS 0–3, mild to moderate disability as diagnostic criteria)(Reference Kurtzke23). Intervention delivery was performed from July 2018 to February 2019.
The study protocol was approved by ethics committee located in the University Medical Sciences and WHO-related Registry of Clinical Trials (IRCT20181113041641N1). The Helsinki ethical principles(24) were well observed throughout the trial. Study objectives were explained, and voluntary informed consent was taken prior to data collection.
Fatigue Severity Scale (FSS), a tool for measuring fatigue in MS, was used to calculate the sample size based on previous reports(Reference Riccio, Rossano and Larocca25).
By the use of sample size determination formula (S1, sem for FSS in control group = 4·73; S2, sem for FSS in intervention group = 4·85;
![]() ${\overline x}_1 - {{\overline x}_2}$
, mean changes for FSS = 3), with a confidence level of 95% (z1 = 1·96), power of 80% (z2 = 1·64) and drop-out rate of 35 % in the number of participants, the total sample size was estimated to be 180.
${\overline x}_1 - {{\overline x}_2}$
, mean changes for FSS = 3), with a confidence level of 95% (z1 = 1·96), power of 80% (z2 = 1·64) and drop-out rate of 35 % in the number of participants, the total sample size was estimated to be 180.
Inclusion and exclusion criteria
Eligible patients had mild to moderate RRMS (defined as EDSS up to 3, and who received dimethyl fumarate 240 mg twice daily in the last year), aged between 20 and 60 years old and ability to write or recall dietary history. Subjects were excluded if they had any of the following: other forms of MS and disease duration of less than 1 year with active relapses, viral infections, such as Epstein Barr, major medical illnesses (such as cancer, allergy, other autoimmune diseases, anticoagulant or antiplatelet use and psychiatric disorders) and current smokers (one or more cigarette per day). Subjects were also excluded if they left more than 40 % blank items on the FFQ at baseline or were prescribed high dose corticosteroid therapy (>30 mg/d methylprednisolone).
Interventions and control groups
The main composition of each diet has been described briefly in Table 1. The intervention group followed a modified version of MeD (mMeD; 17 % protein, 51 % carbohydrate and 32 % fat(Reference Bédard, Riverin and Dodin26)), based on higher intake of fresh fruits and vegetables, whole grains, MUFA, fish and low to moderate consumption of dairy products, meat and poultry. In practice, the prescribed mMeD was individualised based on cultural and personal preferences and the elimination of any alcohol-containing foods and beverages. The control group followed the TID (low in low-fat dairy products, whole grains; high in red meats, solid oils, refined grains and moderate intakes of legumes, fruits and vegetables); based on prior investigations, this diet consisted of 13 % protein, 58 % carbohydrate and 29 % fat(Reference Souri, Amin and Farsam27). It must be noted that the TID group (as control) did not continue their normal eating pattern, i.e. the original dietary principles in the control group were maintained; however, the TID plan was adjusted for energy intake to avoid unexpected body weight changes.
Table 1. The main composition of modified mediterranean (mMeD) and traditional iranian (control) diets
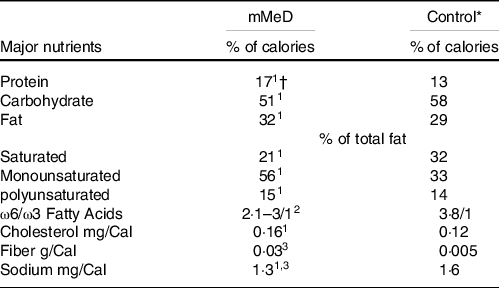
* Values were calculated based on average usual intakes of the participants in Traditional Iranian Diet.
† Reference Number:
1. Bédard A, Riverin M, Dodin S, et al. (2012) Sex differences in the impact of the Mediterranean diet on cardiovascular risk profile. Br J Nutr 108, 1428–1434.
2. Cordain L, Eaton SB, Sebastian A, et al. (2005) Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81, 341–354.
3. Kafatos A, Verhagen H, Moschandreas J, et al. (2000) Mediterranean diet of Crete: foods and nutrient content. J Am Dietetic Assoc 100, 1487–1493.
Modified Mediterranean Diet; adopted from 1999 Greek Dietary Guidelines (1999): Ministry of health and welfare, supreme scientific health council: Dietary guidelines for adults in Greece. Arch. Hell. Med. 1999, 16, 516–524. Serving sizes specified as: 25 g bread, 100 g potato, 50–60 g cooked pasta, 100 g vegetables, 80 g apple, 60 g banana, 100 g orange, 200 g melon, 30 g grapes, 1 cup milk or yoghurt, 1 egg, 60 g meat, 100 g cooked dry beans.
Ideal body weight and the Harris–Benedict equation(Reference Bendavid, Lobo and Barazzoni28) were utilised to calculate the Basal Energy Expenditure for each participant in both diets (mMeD and TID). Next, the above percentages were used to discern the macronutrient requirements in both diets. All the participants received an individualised diet plan, which had been designed according to the above principles. Dietary adherence was also measured with weekly with phone calls and face-to-face interviewing every month.
Recruitment and randomisation methods
Participants were recruited using advertisements in local media outlets and clinicians’ invitation. Participants were randomly assigned into either the modified Mediterranean diet (mMeD; intervention) or TID (control) group, with a computerised random sequence generator. Randomisation was performed by a research assistant who did not participate in either the follow-up assessments or analysis.
Blinding
In this trial, blinding of participants and dietitians is not possible because of obvious differences between the intervention and control diets; however, where appropriate, trial personnel (research assistant who enrolled participants, outcome assessors and data analysts) remained blind to group allocation throughout the study period.
Outcome measurements
The primary outcome was the diet-induced change in DII. The secondary outcomes were change in disease disability (measured by EDSS) and fatigue severity (measured by Modified Fatigue Impact Scale (MFIS)). Socio-demographic and clinical characteristics were collected through a self-report survey completed at baseline, which included details on participants’ age, body weight and height, BMI, education level, family history of MS and supplement use. Baseline DII scores were also assessed in two states: dietary only and dietary plus supplements. However, the statistical analysis was conducted based on dietary DII scores.
Dietary assessment
Food intake of individuals during the previous year was assessed using a validated 168-item semi-quantitative FFQ(Reference Willet and Lenart29), which included a list of foods with standard serving sizes commonly consumed(Reference Esmaillzadeh, Mirmiran and Azizi30–Reference Nematy, Nouri and Ghazizahedi32). Nutritionist IV software (N-squared Computing) was used to analyse the composition of consumed foods. Some DII parameters such as ginger, saffron, turmeric, thyme/oregano and rosemary were additionally added to the FFQ. For calculation of flavonoids (flavan-3-ol, flavones, flavonols, flavonones, anthocyanidins and isoflavones), the USDA Databases for the Flavonoid Content of Selected Foods (Release 3·3, March 2018)(Reference Haytowitz, Wu and Bhagwat33) and Isoflavone Content of Selected Foods (Release 2·0, September 2008)(Reference Bhagwat, Haytowitz and Holden34) were used. Dietary intake of eugenol was estimated according to Phenol-Explorer database (latest version 3·6; released on December 2016)(Reference Yordi, Matos and Martínez35). There were two timepoints for dietary assessment: one before the dietary intervention and one 6 months after the start of the study.
Dietary Inflammatory Index calculation
Shivappa et al. (Reference Shivappa, Steck and Hurley36), after evaluation of 1943 articles (were published between 1950 and 2010), examined the association between inflammation and forty-five food and nutrient parameters; this resulted in the development and validation of DII, where the score ranged from 7·98 (i.e., strongly pro-inflammatory) to −8·87 (i.e. strongly anti-inflammatory). In the present study, we calculated the DII scores at baseline and after 6 months of intervention. Estimated dietary intake data were adjusted against a reference global daily mean and standard deviation intake (from eleven countries)(Reference Shivappa, Steck and Hurley36) for each parameter to obtain a Z-score; each Z-score was converted to percentile, and this value was multiplied by 2 and then subtracted from 1. This number for each intake parameter was multiplied by its respective parameter-specific inflammatory effect score to obtain the parameter-specific DII score. Each of these 45 scores was then summed to obtain an overall DII score.
Fatigue severity assessment
The MFIS was used to determine the MS-related fatigue(Reference Fisk, Ritvo and Ross37) at baseline and 6 months after the intervention. This standard twenty-one item questionnaire has three subscales (physical, ranges from 0 to 36; Cognitive, 0–40 and Psychosocial, 0–8). The total score is computed by summing scores from the three subscales and ranges from 0 to 84, where higher scores represent greater fatigue severity. In the present study, the validated Persian version of MFIS(Reference Harirchian, Nasergivechi and Maddah38), with excellent test-retest reliability(Reference Rietberg, Van Wegen and Kwakkel39), was utilised.
Disability assessment
A trained neurologist measured EDSS to assess MS-related disability(Reference Kurtzke23,Reference Hohol, Orav and Weiner40) at baseline and 6 months after the intervention. Scales for the total EDSS in the current study ranged from 0 (no disability at all) to 3 (mild to moderate disability).
Statistical analysis
Data were presented as means ± sd for continuous variables and number (percent) for categorical variables. The Kolmogorov–Smirnov test was used to assess the normality of continuous variables. In addition, independent student t and paired t tests (or nonparametric Mann–Whitney U and Wilcoxon tests) were used to compare the continuous variables. Categorical variables were compared using the χ2 or Fisher’s exact test. MANCOVA was performed to evaluate the differences for change in DII scores, where the related values were adjusted for age, gender, body weight, BMI, education level, supplement use, family history and duration of MS. The mean changes (Δ) were calculated by subtracting the baseline and 6 months (end) values. To identify the relationship between DII (and other covariates) and fatigue severity/disease activity scores at end of trial, multiple regression analysis was performed. All statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS), version 24 (SPSS Inc.). P < 0·05 was considered to represent statistical significance.
Results
Enrollment and adherence
Between July 2018 and February 2019, we screened 261 RRMS patients; however, sixty-seven subjects were excluded as they did not meet the inclusion criteria, and fourteen patients declined to participate (Fig. 1). In total, 180 patients were dichotomised to the mMeD or TID group. Thirty subjects dropped out during the study follow-up: twenty due to lack of compliance, two due to lack of willingness to continue the study, one due to a driving accident and ten subjects due to incomplete questionnaires. Overall, 147 participant-related data (intervention = 68; control = 79) were analysed (based on per-protocol analysis). No side effects (diarrhoea, abdomen pain, constipation and appetite changes) were reported during the study period.
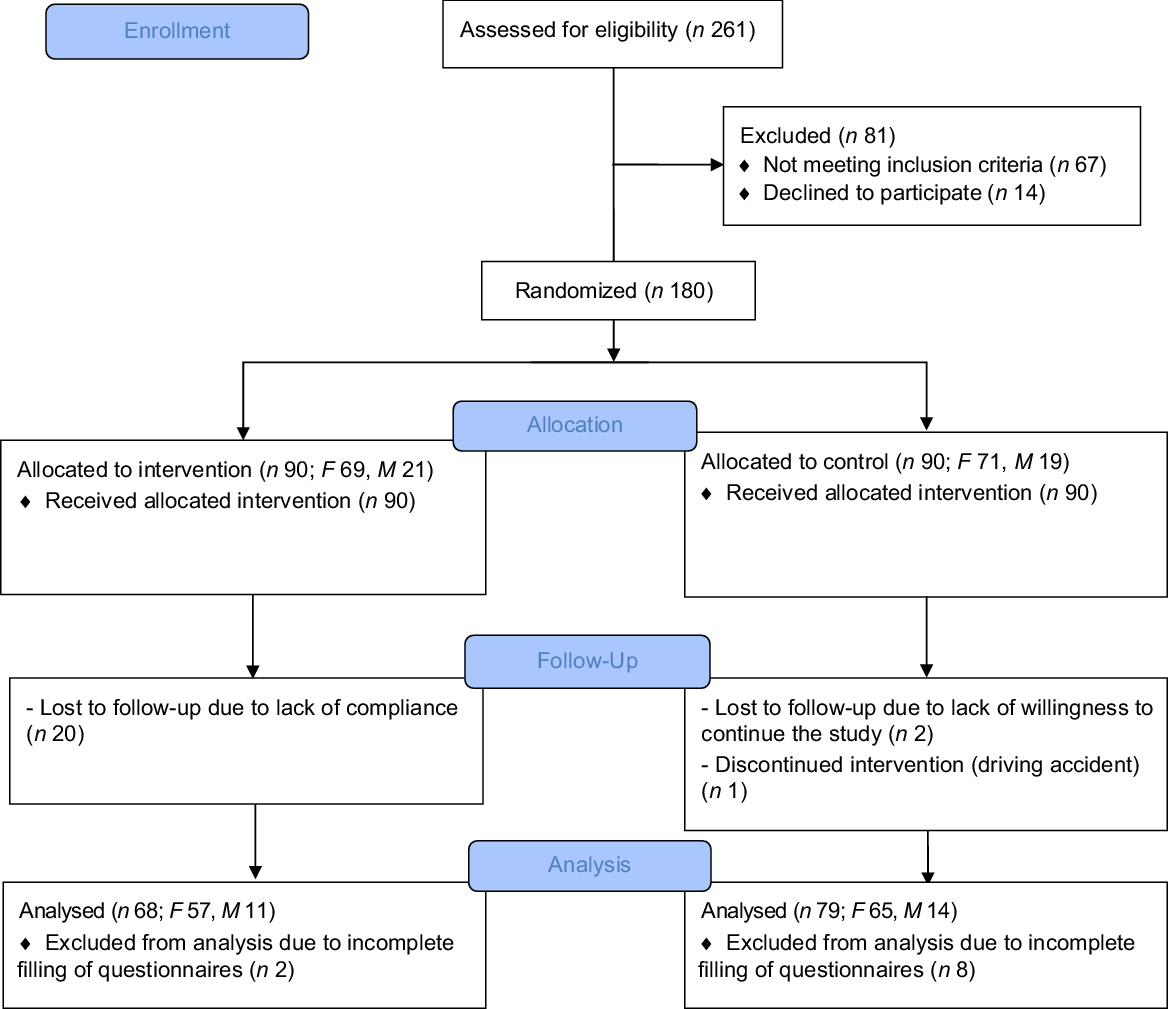
Fig. 1. Flow diagram representing study plan.
Baseline characteristics
Socio-demographic and medical characteristics, between the groups at baseline, are reported in Table 2. Overall, the participants were middle-aged adults (with mean age 39·3 ± 9·2 years old; ˜83% female). More than 40 % were overweight and obese, 15 % had family history of MS and the majority had already completed a degree to diploma level. More than 80 % of the study population were taking at least one type of nutritional supplement, of which vitamin D (˜83 %) and n-3 (˜33%) were the most common. Additionally, ˜20 % of subjects had consumed L-carnitine or caffeine-containing supplements during the past 6 months. A small number of male participants (13 %) were irregular smokers (average 1–2 cigarettes in a week). Mean EDSS score was slightly higher in the control group (2·0 v. 1·7), although there were no significant differences between the study groups for EDSS and DII scores.
Table 2. Characteristics of participants between diet study groups
(Mean values and standard deviations; numbers and percentages)
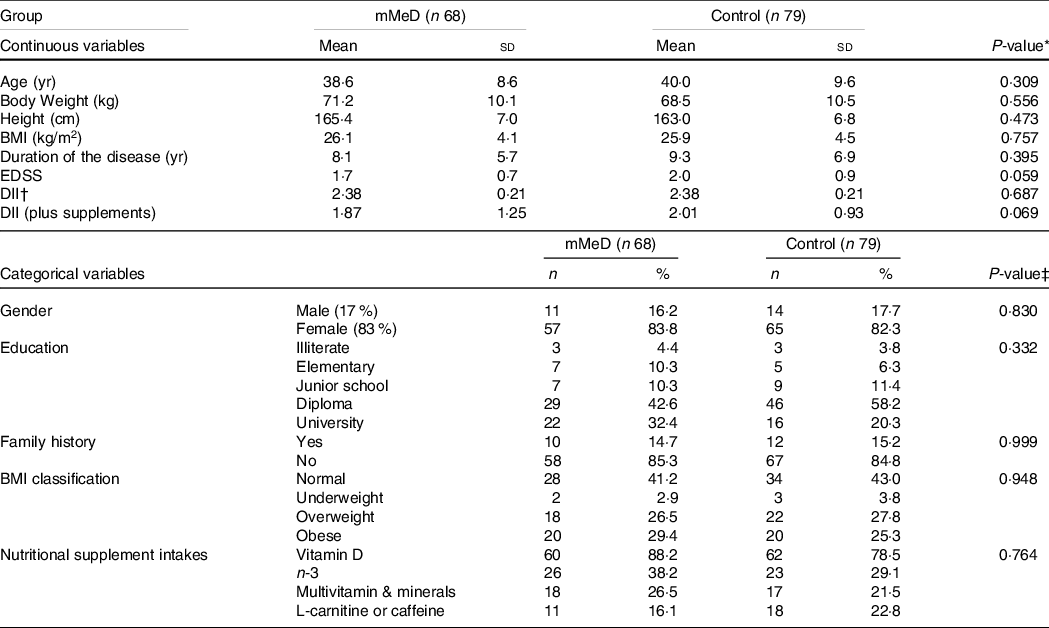
Abbreviations: mMeD, modified Mediterranean Diet; BMI, Body Mass Index; yr, year; sd, Standard Deviation; EDSS, Extended Disability Status Scale; %, within group percent; DII, dietary inflammatory index; control, Traditional Iranian Diet.
* obtained from independent t test, EXCEPT for Duration of the disease, BMI, and EDSS, that was analyzed by Man-Whitney U test; P < 0·05 considered significant.
† determined with Chi Square, EXCEPT for gender, and Family history, that was analyzed by Fisher’s Exact test, P < 0·05 considered significant.
‡ Negative number represents an anti-inflammatory score, while positive number reflects a proinflammatory score.
Clinical outcomes
Impact of diet interventions on Dietary Inflammatory Index
Table 3 details the mean daily intake for 45 DII parameters and overall DII score for each diet. Within the mMeD group, there was a significant decrease from 2·38 ± 0·21 to –1·87 ± 0·86 at 6 months for overall DII score (P < 0·001). Compared with control group (TID), the mean changes for overall DII score were also statistically significant (–4·25 ± 1·54 v. –0·07 ± 0·62; P < 0·001).
Table 3. Dietary inflammatory index (DII) parameters and scores in patients with relapsing-remitting multiple sclerosis that received either modified mediterranean diet (mMeD) or traditional iranian diet (control)
(Mean values and standard deviations)

DII, Dietary inflammatory index; mMeD, modified Mediterranean Diet; control, Traditional Iranian Diet; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Data has been presented as mean ± sd.
Flavonoid intake was estimated based on ‘USDA Database for the Flavonoid Content of Selected Foods; Release 3·2’. Other micro ¯onutrients were calculated with N4 software.
† Obtained from paired t test; except for protein and Vitamin A, that were analyzed via nonparametric Wilcoxon test. P < 0·05 considered as significant.
‡ Mean changes between end of trial and baseline values.
§ Obtained from MANCOVA test; adjusted for age, gender, body weight, body mass index, education level, supplement use, family history and duration of MS, and P < 0·05 considered as significant.
|| Due to the cultural features of Iranian, the intake of alcohol-contained products was close to zero; thus, we modified the standard version of MeD by eliminating any alcohol beverages e.g. red wine.
¶ Calculated from USDA Database for the Flavonoid Content of Selected Foods Release 3·3 (March 2018)
** Calculated from USDA Database for the Isoflavone Content of Selected Foods Release 2·0 (September 2008)
†† Eugenol (mg) intake was measured based on Phenol-Explorer database (latest version 3·6; released on December 2016).
‡‡ Obtained by dietary intakes only. Negative number indicates an anti-inflammatory score, while positive number reflects a proinflammatory score.
For mean daily intake of DII food/nutrient parameters after 6 months (Table 3), there was a significantly higher intake of protein, n-6 fatty acids, MUFA, PUFA, Se, beta carotene, vitamin E, riboflavin, garlic, onion, ginger, turmeric, pepper, thyme/oregano, rosemary, flavan-3-ol, anthocyanidins and isoflavones, in addition to lower intake of energy, carbohydrate, total fat, saturated fat, trans fat, Fe and caffeine in the mMeD group compared with TID group (P < 0·05).
Impact of diet interventions on fatigue
Table 4 details the results for fatigue severity. At the end of the study period, MANCOVA revealed a significant difference between the groups for MFIS total score (Δ for mMeD = −8·5 ± 2·74 v. Δ for TID = 6·4 ± 1·62, P < 0·001). These findings were adjusted for age, gender, body weight, BMI, education level, supplement use, family history and duration of MS.
Table 4. Comparison of fatigue & disability-related variables in patients with relapsing-remitting multiple sclerosis that received either modified mediterranean diet (mMeD) or traditional iranian diet (control)
(Mean values and standard deviations)

MFIS, Modified Fatigue Impact Scale; EDSS, Extended Disability Status Scale; mMeD, modified Mediterranean Diet; control, Traditional Iranian Diet; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
Data has been presented as mean ± sd.
† Obtained from paired t test. P < 0·05 considered as significant.
‡ Mean changes between end of trial and baseline values.
§ Obtained from MANCOVA test; adjusted for age, gender, body weight, body mass index, education level, supplement use, family history and duration of MS, and P < 0·05 considered as significant.
Participants who received mMeD had a statistically significant improvement in physical and cognitive MFIS subscales. After 6 months, there was a 2·7 and 5·6 points reduction in physical (P < 0·001) and cognitive (P = 0·027) MFIS subscales, respectively. However, no significant change in the psychosocial subscale of MFIS was evident.
Impact of diet interventions on disability
There was a nonsignificant reduction in EDSS at the end of the study period in the mMeD group (Δ = −0·02 ± 0·07, P = 0·334). Contrastingly, a nonsignificant rising trend in EDSS was seen in the TID group. MANCOVA, adjusted for age, gender, body weight, BMI, education level, supplement use, family history and duration of MS, did not indicate any significant change for EDSS, in mMeD v. TID (P = 0·065) (Table 4).
Relationship between DII and the fatigue severity/disease disability
Significant predictors and covariates for MFIS total score and EDSS are presented in Table 5. DII score significantly predicted fatigue severity in the intervention group (B = 1·701, P = 0·041; adjusted R2 = 0·098); however, age, gender, body weight, BMI, education level, supplement use, family history and duration of MS had no significant association with MFIS total score.
Table 5. Predictors and covariates for MFIS total score and EDSS in mMeD group
(Coefficient values and 95 % confidence intervals)
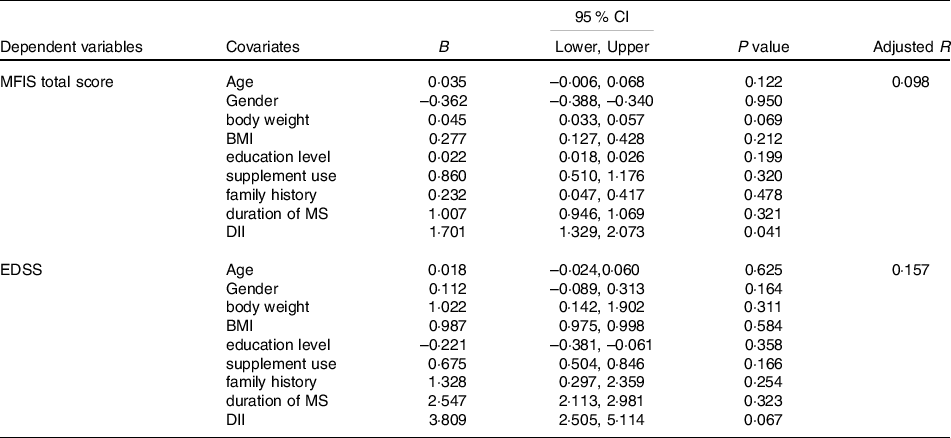
B, unstandardised regression coefficient; 95 % CI, 95 % confidence interval of the unstandardised regression coefficient.
MFIS, modified fatigue impact scale; DII, dietary inflammatory index; EDSS, extended disability status scale; BMI, body mass index; MS, multiple sclerosis‘
In addition, regression analysis revealed that DII score does not predict disease disability in mMeD group (B = 3·809, P = 0·067; adjusted R2 = 0·157). Other covariates (age, gender, body weight, BMI, education level, supplement use, family history and duration of MS) did not show any significant association with EDSS.
Discussion
This study assessed the effects of dietary intervention on DII score in Iranian RRMS patients. Our findings showed that the mMeD possesses significant anti-inflammatory properties, whereas the TID had no significant effect on overall DII score. The key components of DII i.e., MUFA and polyphenols, increased significantly after six months adherence to the mMeD group. Moreover, mMeD also reduced fatigue severity (MFIS score); however, the effect on disease disability (measured by EDSS) was not significant.
Iranian dietary intervention studies typically use TID as the control diet(Reference Paknahad, Sheklabadi and Moravejolahkami11,Reference Goudarzi, Sedaghat and Hedayati41,Reference Moravejolahkami, Paknahad and Chitsaz42) . TID (low in low-fat dairy products, whole grains; high in red meats, solid oils, refined grains and moderate legumes, fruits and vegetables) is the most prevalent diet in Iran and has both positive and negative health-related aspects(Reference Floor43). In a case-control study, conducted by Jahromi et al. (Reference Jahromi, Toghae and Jahromi44), the traditional dietary pattern was inversely related to the risk of RRMS; however, high amounts of red/organ meat in the TID can lead to both neurodegeneration and autoimmune disorders(Reference Agrawal, Berggren and Marks45). Indeed, it must be noted that nutritional transition in Iran has resulted in a change in TID, which must be considered in further research(Reference Floor43,Reference Ghassemi, Harrison and Mohammad46) ; however, the control group adhered to the prescribed TID, as defined in the present study.
In this research, DII was considered as an index that effectively represents dietary inflammatory status, based on several previous studies that have verified by the significant association of DII with inflammatory markers(Reference Zhu, Ling and Mi47). This index presents an alternative assessment tool for inflammation, as opposed to the laboratory-based techniques which are obtained through invasive methods and economically prohibitive(Reference Fowler and Akinyemiju48). Moreover, some previous studies have indicated that the DII may also be correlated with other dietary indices i.e., Healthy Eating Index-2010 (HEI-2010), the Alternative Healthy Eating Index (AHEI) and the Dietary Approaches to Stop Hypertension Index (DASH)(Reference Wirth, Hébert and Shivappa49).
Regardless of the pro-inflammatory DII score for the TID, the mMeD elicited a reduction in the total DII score in the present study. Indeed, the association between MeD and DII score has been evaluated in previous research; for instance, in a Spanish cross-sectional study, adherence to the MeD was higher in the lowest quintile of DII scores(Reference Ruiz-Canela, Zazpe and Shivappa20). Moreover, an inverse correlation was also observed between the DII and MD Score(Reference Hodge, Bassett and Shivappa21), whilst Mayr et al. (Reference Mayr, Thomas and Tierney19) showed that six months MeD adherence, compared with a low-fat diet, elicited an improvement in DII scores in patients with coronary heart disease. Interestingly, Mayr et al. evaluated 45 parameters of DII and reported an anti-inflammatory DII score of −1·74, which is comparable to the total score of −1·87 in the present study. Although we removed the alcohol-containing foods and beverages from our intervention, the current findings were comparable to the intact versions of the MeD reported in the literature.
Recently, it was reported that DII was not significantly associated with the clinical condition of individuals with MS(Reference da Costa Silva, de Carvalho Sampaio and Shivappa50). Moreover, Silva et al. (Reference Silva, Sampaio and Shivappa51), in a cross-sectional study, reported that DII does not correlate to waist circumference, waist-hip ratio, body roundness index, body shape index, body shape z score index and percentage of body fat among MS patients. In contrast, Shivappa et al. (Reference Shivappa, Hebert and Behrooz52) observed that a pro-inflammatory diet (with a higher DII score) may be associated with an increased risk of MS in an Iranian population. In the current study, an anti-inflammatory diet was prescribed to assess the possible effects on DII in RRMS patients. The link between diet and chronic inflammation has been well established(Reference Uccelli and Gattorno53,Reference Sanchez, DePaula-Silva and Libbey54) , and the association between inflammation and neurodegeneration in MS is generally well-supported(Reference Celarain and Tomas-Roig55). According to previous work, the MD is inversely associated with biomarkers of inflammation(Reference Schwingshackl and Hoffmann56,Reference Khalili, Håkansson and Chan57) .
The MUFA and PUFA content of mMeD appears to be responsible for the anti-inflammatory DII score in the current study. n-3 PUFAs inhibit NF-kB signaling through activation of SIRT1-mediated pathway(Reference Inoue, Tanaka and Masuda58) and the reduction of pro-inflammatory cytokines (e.g., IL-12, IL-23)(Reference von Glehn, Dias-Carneiro and Moraes59). Olive oil polyphenols, which are a major part of the mMeD, also have an inhibitory effect on endothelial Nitric Oxide Synthase (eNOS) and Brain-Derived Neurotrophic Factor (BDNF) expression(Reference Zhang, Xue and Hu60). However, two systematic reviews in 2012 and 2020, respectively, reported that PUFAs do not elicit any significant effect on MS-related outcomes(Reference Farinotti, Vacchi and Simi61,Reference Parks, Jackson-Tarlton and Vacchi62) .
In the present study, flavonoids intake increased after six months adherence to the mMeD; moreover, flavan-3-ol, anthocyanidins and isoflavones levels were significantly greater in comparison with the TID group. Flavan-3-ols, mainly extracted from green tea, have previously been advocated as neuroprotective compounds(Reference Zini, Rio and Stewart63). Furthermore, anthocyanidins possess anti-inflammatory and anti-proliferative effects through inhibition of the cyclooxygenase-2 expression in LPS-evoked macrophages(Reference Hou, Yanagita and Uto64). Recently, Freedman et al. (Reference Freedman, Shahi and Zarei65) found that a high-isoflavone diet ameliorates Experimental Autoimmune Encephalomyelitis (EAE) through modulation of gut microbiota in MS patients.
In the present study, fatigue severity was reduced by 12 percent (measured by MFIS total score). In a 12-week randomised trial, Mousavi-Shirazi-Fard et al. (Reference Mousavi-Shirazi-Fard, Mazloom and Izadi13) observed the fatigue-modulatory effect of an anti-inflammatory diet among 100 RRMS patients. Another study, conducted by Yadav et al. (Reference Yadav, Marracci and Kim66), reported that a plant-based, low-fat diet, can reduce the MFIS by ˜0·2 points per month in RRMS patients. Indeed, it seems that the bioactive components of mMeD are responsible for fatigue improvement.
The mMeD administration in the present study did not elicit any improvement in disease-related disability in RRMS participants. We hypothesised that the mMeD may have improved the level of disability from moderate (˜3) to mild disability (≤ 2); however, in this study, and our previous work, there was no association between a Mediterranean-like dietary pattern and disability (measured by EDSS)(Reference Moravejolahkami, Paknahad and Chitsaz14). EDSS is the most important secondary endpoint in MS trials addressing RRMS patients; this instrument is suitable for detecting the efficacy of clinical interventions, to monitor disease progression, and is internationally utilised(Reference Meyer-Moock, Feng and Maeurer67).
While our study provides initial insights into understanding the potential role of dietary interventions in the management of MS, it has some limitations that should be considered. The current study is representative of patients with RRMS undergoing intensive pharmacotherapy, and who are potentially motivated and health conscious. Therefore, the current findings are not necessarily pertinent to healthy subjects, or other disease populations. The sample size for the present study was calculated based on a secondary variable, i.e., FSS. Furthermore, incumbent findings could have been affected by insufficient statistical power, relative to DII score, small sample size, short follow-up period and high drop-out rate (>18%). Moreover, there was an imbalance between groups at follow-up in the drop-out rate (more than 22% in the intervention group v. 3·3 % in the control group). The nature of this study was single blind and was vulnerable to selection and recall biases. The lack of neuroimaging data, which may be useful in evaluating the effect of diet on neurodegeneration, was the most important clinical limitation. The calculation of trans fat intake was predominantly based on high-fat dairy and meat products; thus, underestimation was possible. Finally, EDSS was used to measure disease disability in the current research; however, this tool may not be sensitive to clinical change, especially in short-term studies (≤ 6 months) and milder levels of disability(Reference Hohol, Orav and Weiner68).
However, despite the aforementioned limitations, the present study has several strengths worth mentioning. The MS participants recruited were relatively homogenous, allowing pertinent inferences to be drawn. Furthermore, adjustment in the final analysis allowed detailed consideration of potential confounding variables. In this trial, all forty-five parameters of the DII were measured, and a non-invasive method was used for evaluating inflammatory condition; these strategies helped to improve the accuracy and precision of our findings. Finally, although current evidence suggests that adherence to a Mediterranean-style diet can reduce inflammation in chronic diseases, studies pertaining to RRMS are limited; therefore, the present study provides a novel and important addition to the literature.
Conclusion
Our results demonstrated that adherence to the mMeD for 6 months can reduce DII score in RRMS participants. Indeed, the mMeD improved fatigue severity, without any significant change in disability. Comparatively, adherence to the TID did not impact DII scores. Additional studies are required to evaluate the long-term safety and immunomodulatory properties of the MeD, and TID in progressive forms of MS, as well as in patients with other autoimmune diseases.
Acknowledgements
The authors thank all the participants of the study for their enthusiastic of involvement and to the personnel of the clinic. This research was supported by Student Research Committee of Isfahan University of Medical Sciences, Isfahan, Iran (Grant NO. 197140).
The authors declare no support from any commercial organisation for the submitted study.
The authors declare that there is no conflict of interest.
CRediT author statement
Jalal Bohlouli: Investigation, Funding acquisition; Iman Namjoo: Visualization, Project administration; Mohammad Borzoo-Isfahani: Data Curation, Resources; Fariborz Poorbaferani: Formal analysis; Amir Reza Moravejolahkami: Conceptualization, Methodology, Software, Validation, Formal analysis; Writing - Review & Editing, Supervision; Cain C. T. Clark: Writing - Review & Editing; Mohammad Ali Hojjati Kermani: Writing - Original Draft.









