Introduction
Ellescus Dejean, 1821 (Coleoptera: Curculionidae: Ellescini) is Holarctic in distribution, with representatives in Canada, the United States of America, across Europe, Russia, Japan (Alonso-Zarazaga and Lyal Reference Alonso-Zarazaga and Lyal1999), Korea (Han and Park Reference Han and Park2015), and China (Su et al. Reference Su, Huang, Omar, Ren, Alonso-Zarazaga, Majer and Zhang2012). Members of the genus are associated with the closely related north-temperate plant genera Salix Linnaeus (Salicaceae) and Populus Linnaeus (Salicaceae) (Anderson Reference Anderson, Arnett, Thomas, Skelley and Frank2002), and the larvae are known to develop in female catkins (Scherf Reference Scherf1964). Pupation takes placed in the soil, after the catkins have fallen to the ground (Hoffmann Reference Hoffmann1954; Scherf Reference Scherf1964). Species of Ellescus exhibit moderate sexual dimorphism externally; males possess medially concave first and second ventrites (not noticeably concave in females) and a comparatively shorter rostrum (longer in females) and have the last two tergites sclerotised (only the last tergite is sclerotised in females; Hoffmann Reference Hoffmann1954; Anderson Reference Anderson, Arnett, Thomas, Skelley and Frank2002). Ellescus is currently placed in the tribe Ellescini with several other genera, including the Holarctic genus Dorytomus Germar, 1817 and the North America endemic genus Proctorus LeConte, 1876 (Alonso-Zarazaga and Lyal Reference Alonso-Zarazaga and Lyal1999; Caldara et al. Reference Caldara, Franz, Oberprieler, Leschen and Beutel2014). Ellescus is easily differentiated from those related willow-feeding taxa: Ellescus possesses basally toothed tarsal claws and untoothed femora, whereas Dorytomus has simple tarsal claws and Proctorus has toothed femora (Anderson Reference Anderson, Arnett, Thomas, Skelley and Frank2002).
The first use of the genus name Ellescus is attributed to Dejean (1821), who listed nine species, two of which are still considered valid – namely, E. bipunctatus (Linnaeus, 1758) and E. scanicus (Paykull, 1792). Species of Ellescus commonly appear under the alternate spelling “Elleschus”; however, this is an unjustified emendation by Schoenherr (Reference Schoenherr1838; Alonso-Zarazaga and Lyal Reference Alonso-Zarazaga and Lyal1999; Alonso-Zarazaga et al. Reference Alonso-Zarazaga, Barrios, Borovec, Bouchard, Caldara and Colonnelli2017). Before the present study, nine Ellescus species were recognised (O’Brien and Wibmer Reference O’Brien and Wibmer1982; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013; note that Franz and O’Brien (Reference Franz and O’Brien2001) subsequently transferred the ninth species, E. carludovicae (Guenther, 1936), to Perelleschus Wibmer and O’Brien, 1986 on morphological grounds), four of which were recorded from North America (E. bipunctatus, E. borealis (Carr, 1920), E. ephippiatus (Say, 1831), and E. scanicus). No comprehensive revision of the genus has been undertaken, and Anderson (Reference Anderson, Arnett, Thomas, Skelley and Frank2002) noted that the species of Ellescus in North America require taxonomic revision. Here, we use a combination of morphological and molecular techniques to review the species of Ellescus in North America. Methodology includes examination of external morphology, dissection of the genitalia of both sexes, and analysis of DNA sequences (CO1, ITS2).
Methods
Specimen acquisition and morphological analyses
Specimens were borrowed from museum and university insect collections via cross-institutional loans. Institution names and associated acronyms used in this work are as follows:
-
AFCF: Atlantic Forestry Centre, Canadian Forest Service, Natural Resources Canada, Fredericton, New Brunswick, Canada
-
CAS: California Academy of Sciences, San Francisco, California, United States of America
-
Centre for Biodiversity Genomics, Guelph, Ontario, Canada
-
CMNC: Canadian Museum of Nature, Ottawa, Ontario, Canada
-
CNCI: Canadian National Collection of Insects, Ottawa, Ontario, Canada
-
CUAC: Clemson University Arthropod Collection, Clemson, South Carolina, United States of America
-
DEBU: University of Guelph Insect Collection, Guelph, Ontario, Canada
-
JHLC: Jake H. Lewis Collection, Tancha, Okinawa, Japan
-
LSAM: Louisiana State Arthropod Museum, Baton Rouge, Louisiana, United States of America
-
MEM: Mississippi State Entomology Museum, Mississippi, Mississippi, United States of America
-
MIZ: Museum of the Institute of Zoology, Polish Academy of Sciences, Warsaw, Masovia, Poland
-
NBM: New Brunswick Museum, Saint John, New Brunswick, Canada
-
RAM: Royal Alberta Museum, Edmonton, Alberta, Canada
-
RBCM: Royal British Columbia Museum, Victoria, British Columbia, Canada
-
RWC: Reginald Webster Collection, Charters Settlement, New Brunswick, Canada
-
UAIC: University of Arizona Insect Collection, Tuscon, Arizona, United States of America
-
UAM: University of Alaska Museum Insect Collection, Fairbanks, Alaska, United States of America
-
USNM: United States National Museum, Washington, District of Columbia, United States of America
-
ZMUO: University of Oulu Zoological Museum, Oulu, North Ostrobothnia, Finland
-
ZSM: Zoologische Staatssammlung München, München, Bavaria, Germany
Specimens were also procured through targeted collecting efforts in eastern Canada and Slovakia. These efforts concentrated on beating branches of Salix and Populus and on sifting leaf litter at the base of plants from mid-April to mid-July in 2020 and 2021. Field-collected specimens were placed in individual vials (i.e., one specimen per vial) with 95% ethanol and stored in a freezer at –23 °C until DNA extraction. Specimens were dissected using standard procedures, and genitalia were cleared using a solution of water and potassium hydroxide (KOH). The cleared genitalia were placed in vials with glycerin and attached to the pin with the associated specimen. All images were taken using a Leica Z16 APOA camera and stacked using LAS image software (Leica Microsystems, Wetzlar, Germany).
Molecular techniques
Specimens collected from the field and stored in 95% ethanol in a freezer at –23°C produced high-quality sequence data using methods outlined in Ivanova et al. (Reference Ivanova, deWaard and Hebert2006). When extracting from older dried museum specimens (pointed), however, Ivanova et al.’s (Reference Ivanova, deWaard and Hebert2006) methods did not produce satisfactory sequence data. In these cases, the whole-insect extraction methods of Santos et al. (Reference Santos, Ribeiro, Cabral and Speranca2018) were used instead. To complement the morphological analysis of Ellescus, sequence data from the mitochondrial protein–coding gene CO1 and the second internal transcribed spacer ITS2 (nuclear rDNA) were obtained using standard polymerase chain reaction and Sanger sequencing methods. Although the widely employed barcode gene CO1 accurately separates taxa in many cases, it fails in others (e.g., Elias et al. Reference Elias, Hill, Willmott, Dasmahapatra, Brower, Mallet and Jiggins2006; Whitworth et al. Reference Whitworth, Dawson, Magalon and Baudry2007). To account for this possibility, ITS2 sequence data were also used because it has been successfully applied in multiple beetle groups (e.g., Gallego and Galian Reference Gallego and Galian2001; Fossen et al. Reference Fossen, Ekrem, Nilsson and Bergsten2016) and, as a faster-evolving region in many groups, it can serve as a finer measure for delineating species (Navajas et al. Reference Navajas, Legnel, Gutierrez and Boursot1998; Skevington Reference Skevington2005). The general polymerase chain reaction recipes, thermal profiles, and primers used are noted in Supplementary material, Tables S1. Polymerase chain reaction products were visualised using gel electrophoresis, and double-stranded sequencing was performed on an ABI 3500XL sequencer (Applied Biosystems™, Thermo Fisher Scientific, Waltham, Massachusetts). Sequence editing, alignment, and analyses were performed in Geneious Prime, version 2021.1.1 (Dotmatics, Boston, Massachusetts, United States of America, https://www.geneious.com). As a precautionary step, all sequences were run through BLAST (Altschul et al. Reference Altschul, Gish, Miller, Myers and Lipman1990) to ensure that nontarget DNA had not been amplified. All DNA sequence data used in this study were uploaded to GenBank (see Supplementary material, Table S2). Alignment of sequences was performed in MUSCLE, version 3.8.425 (Edgar Reference Edgar2004) using default settings, and neighbour-joining trees were constructed with 10 000 bootstrap replicates for both markers under a Jukes–Cantor substitution model with a 70% support threshold. Tree stylisation was enhanced in FigTree, version 1.4.4 (Rambaut Reference Rambaut2009).
Results and discussion
Taxonomy
A taxonomic investigation of the North American members of the genus Ellescus revealed the presence of four species on the continent, each with unique external and genital (male and female) morphology, as well as DNA sequences (CO1, ITS2; Figs. 1, 2). This includes E. ephippiatus (Say, 1831), the Holarctic E. bipunctatus (Linnaeus, 1758) (of which E. borealis (Carr, 1920) new synonym is a new junior synonym), the west coast endemic E. californicus (Casey, 1885) (resurrected from synonymy with E. ephippiatus (Say, 1831)), and the temperately distributed E. michaeli new species. The European species, E. scanicus (Paykull, 1792), is determined to have been erroneously reported from North America. The fifth ventrite of males bear modifications (carinae, tubercles) that are taxonomically important and allow for separation of the species into groups. Furthermore, aedeagus morphology and, to a lesser extent, spermatheca morphology are important and sometimes necessary for reliable species identification. Species profiles and a photographic key to the species (including E. scanicus) are presented below.

Fig. 1. Neighbour-joining tree (CO1) for Ellescus species under a Jukes–Cantor substitution model (10 000 bootstrap replicates, support threshold of 70%). Note that Ellescus ephippiatus and Ellescus michaeli new species do not separate when CO1 is used but do separate when the faster-evolving marker ITS2 is used (see Fig. 2).

Fig. 2. Neighbour-joining tree (ITS2) for Ellescus species under a Jukes–Cantor substitution model (10 000 bootstrap replicates, support threshold of 70%).
Ellescus Dejean, 1821
Ellescus Dejean, 1821; Alonso-Zarazaga and Lyal Reference Alonso-Zarazaga and Lyal1999: 78; Anderson Reference Anderson, Arnett, Thomas, Skelley and Frank2002: 738; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013: 326; Caldara et al. Reference Caldara, Franz, Oberprieler, Leschen and Beutel2014: 609; Alonso-Zarazaga et al. Reference Alonso-Zarazaga, Barrios, Borovec, Bouchard, Caldara and Colonnelli2017: 196.
Alyca LeConte, 1876.
Anisarctus Desbrochers des Loges, 1907.
Elleschus Schoenherr, Reference Schoenherr1838 (unjustified emendation); Hubbard et al. Reference Hubbard, Schwarz and LeConte1878: 621; Austin Reference Austin1880: 49; Hamilton Reference Hamilton1889: 156; Slosson Reference Slosson1897: 239; Blatchley and Leng Reference Blatchley and Leng1916: 283; Hoffmann Reference Hoffmann1954: 1131; O’Brien and Wibmer Reference O’Brien and Wibmer1982: 116; Mattson et al. Reference Mattson, Niemela, Millers and Inguanzo1994: 10; Ciegler Reference Ciegler2010: 71; Prena Reference Prena2018: 382.
Sarapus Villa and Villa, 1833.
Gender. Masculine.
Type species. Curculio scanicus (Paykull, 1792) (by subsequent designation (Schoenherr Reference Schoenherr1838: 321); see O’Brien and Wibmer (Reference O’Brien and Wibmer1982) and Alonso-Zarazaga and Lyal (Reference Alonso-Zarazaga and Lyal1999)).
Redescription. Length 1.9–3.0 mm. Small, elongate-oval, cuticle dark, brown, yellow, dull orange, and/or dull red. Cuticle covered in coarse, white, and/or yellow hair-like or broader scales. Rostrum stout, roughly equal in length to pronotum, and often covered in scales up to antennal insertion. Eyes small, circular to oval in shape, extending somewhat onto the rostrum medially. With or without prominent fovea between eyes. Antennae reddish-brown with small, oval-shaped club. Pronotum as wide as long, coarsely punctate, scaled, with or without prominent smooth, longitudinal midline. Scutellum usually covered with white scales, often appearing brighter than surrounding regions of elytra. Elytra oval-shaped in dorsal view, striae with large, deep punctures, each bearing a scale. Punctures of elytral striae distinctly larger than those of pronotal disk. Interstrial regions of elytra with 2–3 irregular rows of scales. In lateral view, elytra largely straight in first half to two-thirds of length, more curved in apical third. Ventrites flat, punctate, covered in scales. First 2–3 ventrites depressed medially in male, unmodified in female. Last ventrite, with glabrous, longitudinal carina or swelling at apex in male, unmodified in female. Legs with femora not toothed (except in E. scanicus, which occasionally bears a minute tooth). Tibiae straight, slightly shorter than femora. Preapical tarsomere bi-lobed. Tarsal claws bearing basal tooth. Aedeagus rounded, subquadrate, or pointed at apex. Internal sac with or without protruding V-shaped basal structures.
Key to the species of Ellescus in North America (including E. scanicus)
-
* Note that E. scanicus was previously reported from North America; however, this is apparently erroneous because all examined Nearctic specimens labelled as E. scanicus were incorrectly identified. The species is included here for reference.
-
0a) First two ventrites depressed medially; apical with ventrite shiny, medial carina extending longitudinally, or with distinct medial swelling at tip (occasionally reduced or totally absent; Fig. 3A, B). Apical two tergites sclerotised (viewing this character often requires relaxing and manipulating specimens). Rostrum shorter[Male]

Fig. 3. Morphological characters used to distinguish Ellescus in North America: A, glabrous carina extending longitudinally across last ventrite (male E. bipunctatus); B, swelling at the tip of last ventrite (male E. californicus, E. ephippiatus, and E. michaeli new species); C, metaventrite and metepisternum with dense, heavily appressed scales covering cuticle (found only in both sexes of E. scanicus); and D, metaventrite and metepisternum without dense, heavily appressed scales (Ellescus species other than E. scanicus).
-
0b) First two ventrites not distinctly depressed medially; apical ventrite unmodified. Only apical tergite sclerotised. Rostrum longer[Female]
-
1a) Males with glabrous carina extending longitudinally over middle of apical ventrite; not prominent at apex of ventrite (Fig. 3A). Metepisternum and lateral half of metaventrite densely punctate and covered with appressed white–yellow scales (Fig. 3C), or with metepisternum and lateral half of metaventrite not densely punctate or scaled, with cuticle in those regions mostly visible (Fig. 3D). Pronotum rarely with regions of discoloured scales laterally (Fig. 4). Apex of aedeagus well rounded or subquadrate, not pointed (Fig. 5A, B). Internal sac without fully protruding basal V-shaped structures (Fig. 5A, B). Spermatheca without prominent basal projection (Fig. 6A, B) 2

Fig. 4. Ellescus bipunctatus (Linnaeus, 1758): A, lateral view of “scanicus” form; B, dorsal view of “scanicus” form, with alternating bands of white scales and strong band along elytral suture; C, lateral view of red and black specimen; D, dorsal view of red and black specimen; E, lateral view of dark specimen; and F, dorsal view of dark specimen.

Fig. 5. Aedeagi of Ellescus species: A, Ellescus scanicus; B, Ellescus bipunctatus; C, Ellescus californicus; D, Ellescus michaeli new species; and E, Ellescus ephippiatus.

Fig. 6. Spermatheca of Ellescus species: A, Ellescus scanicus; B, Ellescus bipunctatus; and C, Ellescus californicus. Note that E. ephippiatus and E. michaeli new species are not presented because the spermatheca of these species do not differ noticeably from that of E. californicus.
-
1b) Males with prominent swelling at apex of apical ventrite [reduced in some smaller specimens] (Fig. 3B) or without any swelling. Metepisternum and lateral half of metaventritenum not as densely punctate or scaled, cuticle in those regions mostly visible (Fig. 3D). Pronotum with regions of discoloured scales laterally [occasionally reduced or absent completely] (Figs. 7–9). Apex of aedeagus ending in a rounded to sharp point (Fig. 5C–E). Internal sac with fully protruding basal V-shaped structures (Fig. 5C–E). Spermatheca with prominent basal projection (Fig. 6C) 3

Fig. 7. Ellescus californicus (Casey, 1885): A, dorsal view and B, lateral view.

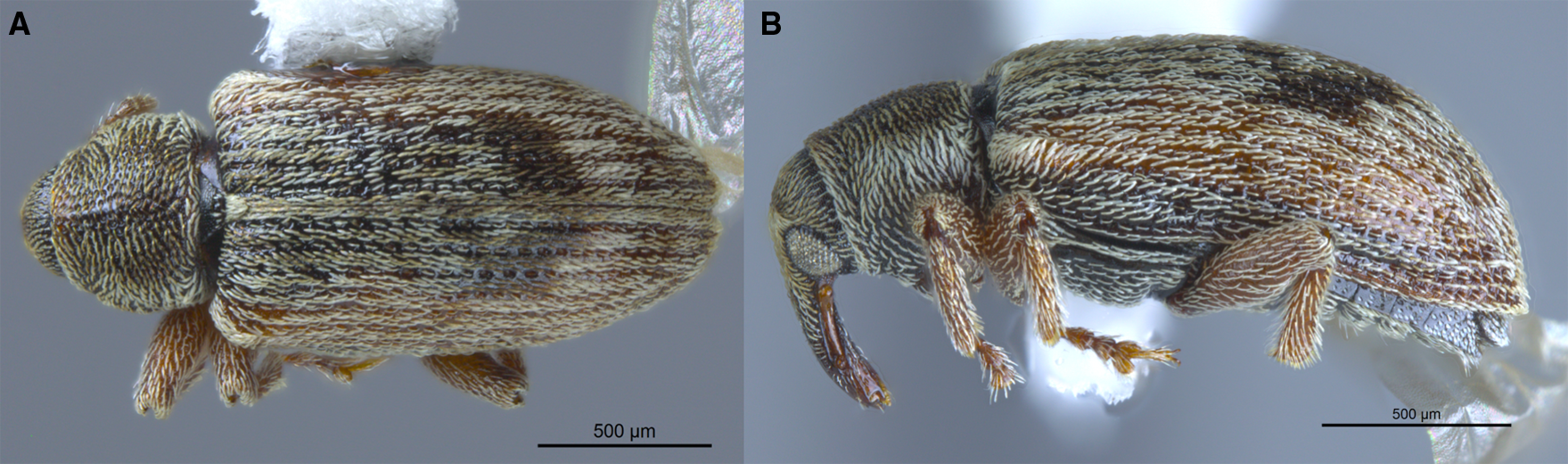
Fig. 8. Ellescus michaeli new species: A, dorsal view of typical specimen; B, lateral view of typical specimen; C, dorsal view of all-red specimen from Otter Tail Co., Minnesota, United States of America; and D, lateral view of all-red specimen from Otter Tail Co., Minnesota.

Fig. 9. Ellescus ephippiatus (Say, 1832): A, dorsal view of red specimen with conspicuous pattern of scales on elytra; B, lateral view of red, patterned specimen; C, dorsal view of piceous, dark specimen; D, lateral view of piceous, dark specimen; E, dorsal view of red and black specimen lacking conspicuous pattern on elytra; and F, lateral view of red and black specimen.
-
2a) Metepisternum and lateral half of metaventrite densely punctate and covered with appressed white–yellow scales, cuticle in those regions mostly concealed (Fig. 3C). Apex of aedeagus subquadrate (Fig. 5A). Internal sac with distinct V-shaped structure. (Fig. 5A). Spermatheca smoothly curved, without basal sinuation and inwards pointing tip (Fig. 6). E. scanicus (Paykull, 1792) (European; not present in North America)
-
2b) Metepisternum and lateral half of metaventrite not as densely punctate or scaled, cuticle in those regions mostly visible and with interspaces shiny (Fig. 3D). Apex of aedeagus rounded (Fig. 5B). Internal sac lacking V-shaped structure (instead with hook-like structure and roughened tissue; Fig. 5B). Spermatheca not smoothly curved but with basal sinuation and often with inward curved tip (Fig. 6B)E. bipunctatus (Linnaeus, 1758)
-
3a) Cuticle mixed in colour (red, black, yellow, brown; Figs. 7, 8A, B, 9) 4
-
3b) Cuticle entirely red or pale yellow (Fig. 8C, D) 6 [Dissection of males usually required]
-
4a) Metaventrite not contrasting with black femora, tibiae, and rostrum (Fig. 8A, B). Elytral colouration diagnostic: dark with transverse bright orange band in apical half (Fig. 8A, B). Apex of aedeagus ending in broadly rounded tip; lateral edges of apex dorsoventrally flattened, with setae distributed along surface (Fig. 5D). Length 1.9–2.2 mm Ellescus michaeli Lewis and Anderson, new species (in part)
-
4b) Metaventrite black and contrasting with reddish-brown legs and apical half of rostrum (Figs. 7, 9). Elytral colouration variable but never as above (Figs. 7, 9). Apex of aedeagus more pointed; apex with two prominent tufts of setae (Fig. 5C, E). Length 2.0–2.9 mm 5
-
5a) Apex of aedeagus quadrate to rounded, dorsoventrally flattened, and ending in an abrupt, sharp point; lateral edges of aedeagus heavily sclerotised (Fig. 5C). Restricted to California and southern Oregon Ellescus californicus (Casey, 1885) (in part)
-
5b) Apex of aedeagus rounded, not distinctly dorsoventrally flattened, and ending in a distinct point; lateral edges of aedeagus not as heavily sclerotised (Fig. 5E). Widespread across North America (although apparently absent from California and southern Oregon) Ellescus ephippiatus (Say, 1831) (in part)
-
6a) Apex of aedeagus ending in broadly rounded tip; lateral edges of apex dorsoventrally flattened, with setae distributed along surface (Fig. 5D). Length 1.9–2.2 mm Ellescus michaeli Lewis and Anderson, new species (in part)
-
6b) Apex of aedeagus ending in sharp tip (Fig. 5C–E). Length 2.0–2.9 mm7
-
7a) Apex of aedeagus quadrate and coming to a distinct, sharp point; lateral edges heavily sclerotised (Fig. 5C). Restricted to California and southern Oregon Ellescus californicus (Casey, 1885) (in part)
-
7b) Apex of aedeagus rounded, ending in a distinct point; lateral edges not heavily sclerotised (Fig. 5E). Widespread across North America (although apparently absent from California and southern Oregon) Ellescus ephippiatus (Say, 1831) (in part)
Ellescus bipunctatus (Linnaeus, 1758)
Curculio bipunctatus Linnaeus, 1758 (original description); Paykull Reference Paykull1792: 58 (description, comment).
Curculio unipunctatus Olivier, 1791 (description).
Orchestes ruficornis Zetterstedt, 1837 (description).
Mecinus erythrocerus Abeille de Perrin, 1910 (description).
Elleschus bipunctatus; Hubbard et al. Reference Hubbard, Schwarz and LeConte1878: 621 (taxonomic comment); Austin Reference Austin1880: 49 (catalogue); Hamilton Reference Hamilton1889: 156 (distribution); Slosson Reference Slosson1897: 239 (checklist); Blatchley and Leng Reference Blatchley and Leng1916: 283 (identification, distribution); Hoffman Reference Hoffmann1954: 1131 (identification); O’Brien and Wibmer Reference O’Brien and Wibmer1982: 116 (catalogue, distribution); Mattson et al. Reference Mattson, Niemela, Millers and Inguanzo1994: 10 (catalogue).
Elleschus bipustulatus; Hatch Reference Hatch1971: 342 (error; catalogue).
Elleschus borealis Carr, 1920 (description); Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013: 326 (catalogue, distribution); new synonym.
Ellescus bipunctatus; Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013: 326 (catalogue, distribution); Webster et al. Reference Webster, Anderson, Webster, Alderson, Hughes and Sweeney2016: 373–374 (distribution); Alonso-Zarazaga et al. Reference Alonso-Zarazaga, Barrios, Borovec, Bouchard, Caldara and Colonnelli2017: 196 (catalogue, distribution).
Notes on the type series. This species was described from an unknown number of specimens collected in “Europa”. Type specimens of this species are not present in the Linnaean Society of London collections or the University of Uppsala’s Museum of Evolution collection and are therefore presumed lost. Although all specimens of E. bipunctatus examined in European collections were accurately identified and the “consensus identity” of this species is relatively stable in Europe, North American specimens of this species frequently have been confused with E. scanicus (see Fig. 4A, B), supporting the designation of a neotype for this species. Here, we designate a male ZSM specimen as the neotype to properly stabilise the identity of this species. We chose the ZSM specimen (Germany) because the original specimens used to describe the species were European in origin and dark in colour. The neotype specimen has, attached, a rectangular red label reading “NEOTYPE” with the species name, species author, and year of description, and a line reading “designated Lewis and Anderson” along with the year of designation.
Specimens examined
Neotype (designated here). GERMANY: Bavaria, Holzkirchen, 8.v.1959, K. Gaigl (male, ZSM).
Other material. AUSTRIA: Lower Austria, Tullnerbach, A. Winkler (2, CMNC); Wendbach (1, MIZ), COL000400; CANADA: Alberta: Calgary, 14.vi.1953–18.vi.1959, B. and J. Carr (8, CNCI); Ghost Dam, 18.vi.1959–22.v.1981, B. and J. Carr (7, CNCI); Edmonton, vi.1915–26.iv.1922, F.S. Carr (3, CNCI, 2, CAS; 6, MCZC), MCZ-ENT00726981, MCZ-ENT00726982, MCZ-ENT00726985 (E. borealis PARATYPE), MCZ-ENT00727085, MCZ-ENT00727188, MCZ-ENT00727189; Edmonton, 10.v.1916, A.C. Davis (4, CNCI); 24 mi. S of Lloydminster, 30.v.1963, Forest Insect Survey, on Populus tremuloides (1, CNCI); McMurray, 4.vi.1953, Brooks–Wallis (1, CNCI); Exshaw, 27.vi.1954, B.F. and J.L. Carr (1, CNCI); Manitoba: Aweme, 28.iv.1903–19.v.1922, N. Criddle (1, DEBU; 1, CNCI; 1, MCZC), DEBU01089104, MCZ-ENT00726983; “Manitoba” (1, RBCM), RBCM EENT991-111634; Gillam, 11.vi.–17.vii.1950, W.J. Brown and J.F. McAlpine (27, CNCI); Onanole, Riding Mountain National Park, 8.vi.1937–15.vi.1938, W.J. Brown (15, CNCI); Carberry, 9.v.1953, Brooks–Kelton (1, CNCI); Makinak (3, CNCI); New Brunswick: Queens Co., Cranberry Lake P.N.A. (46.1125° N, 65.6075° W), 3–25.v.2011, M. Roy and V. Webster, Red Oak forest Lindgren funnel trap (2, NBM), NBM-070113 – NBM-070115; Belleville Meduxnekeag Valley Nature Preserve (46.1878° N, 67.6705° W), 18.viii.2008, R.P. Webster (1, NBM), NBM-070105; Northwest Territories (new territorial record): Norman Wells, 9.iv.1949–26.v.1953, S.D. Hicks and C.D. Bird (75, CNCI); Ontario: Ridgeway, Liebeck (3, MCZC), MCZ-ENT00726946, MCZ-ENT00726947, MCZ-ENT00727187; Prince Edward Co., 28.v.1919–1.vi.1923, J.F. Brimley (11, CNCI); Rainy River District, 14.v.1924, J.F. Brimley (6, CNCI); Emo, 21.vi.1924, J.F. Brimley (2, CNCI); Constance Bay, 6.viii.1959, H. Howden, on sweetfern (1, CNCI); Constance Bay, 17.iv.1968–29.iv.1998, Henry and Anne Howden (60, CMNC); Constance Bay, 1970, S. Peck (1, CNCI); Constance Bay, 21.iv.1977, M. Sanborne (2, CMNC); Carleton Co., Constance Bay, 26.iv.1935, W.J. Brown (5, CNCI); Constance Bay, 19.v.1933, W.J. Brown (22, CNCI); Prince Edward Co., 14.viii.1964, J.F. Brimley (4, UAIC), UAIC1072869; Ottawa, 22.iv.1976, M. Sanborne, on willow (2, CMNC); Ottawa, 23.v.1965, A.T. Howden (1, CMNC); Ottawa, 6.v.1930, W.J. Brown (3, CNCI); Ottawa, 17.v.1961, Kelton (1, CNCI); Mer Bleue, 13.v.1932–10.v.1933, W.J. Brown (38, CNCI); Kenora District, Minnitaki, 12.v.1960, F.I.S., on female Populus tremuloides catkins (2, CNCI); Hartington, Eel Lake, South Frontenac (44.563° N, 76.5522° W), 6.ix.2017, J. deWaard (1, CBG), BIOUG35446-H11, ELPCH211–17; Hartington, Eel Lake, South Frontenac (44.5645° N, 76.5516° W), 25.vii–8.viii.2017, G. Blagoev (1, CBG), BIOUG35442-G10, ELPCG9470-17; Quebec: Cantley, 45.591726° N, 75.784856° W, 17.v.2020, J.H. Lewis, (1, CMNC), LEW_69, OP215778 (GenBank CO1 accession number), OP218943 (GenBank ITS2 accession number); near chemin Holmes (Cantley), 45.598843° N, 75.798396° W, on female Salix catkins, J.H. Lewis, (1, CMNC), LEW_71, OP215779 (GenBank CO1 accession number), OP218944 (GenBank ITS2 accession number); Cantley, 45.59468° N, 75.794389° W, 24.iv.2021, J.H. Lewis, beaten of Salix sp., (1, CMNC), LEW_135, OP215784 (GenBank CO1 accession number), OP218954 (GenBank ITS2 accession number); Rigaud, Liebeck Collection (1, MCZC), MCZ-ENT00726952; Gatineau, near Boucher Forest (45.427673° N, 75.823561° W), 2.v.2021, J.H. Lewis, beaten from Populus tremuloides (2, CMN), LEW_118, OP215783 (GenBank CO1 accession number); Gatineau, Mont Cascades (45.588793° N, 75.845822° W), 13.v.2021, J.H. Lewis, beaten from Salix (1, CMNC), LEW_90, OP215780 (GenBank CO1 accession number), OP218948 (GenBank ITS2 accession number); Estrie Region, Scotstown, 14–21.v.2012, C. Levesque (2, CNCI); Kirks Ferry, 25.v.1950, B.P. Beirne, in light trap (1, CNCI); Laniel, 14.viii.1982, W.J. Brown (1, CNCI); Gatineau Park, Harrington Lake, 31.v.1954, R. McCondochie (1, CNCI); Cartier, Mount Jacques, 9.vii.1954, W.J. Brown (1, CNCI); Kazubazua, 25.v.1933, W.J. Brown (8, CNCI); Seven Islands, 8.vi.1929, W.J. Brown (6, CNCI); Saskatchewan (new provincial record): Prince Albert, 3.vii.1954, Brooks–Wallis (1, CNCI); Fish Creek, 20.v.1928, K.M. King (3, CNCI); Elbow, 23.vi.1954, Brooks–Wallis (1, CNCI); FINLAND: Espoo, Vanttila (60.1829° N, 24.6157° E), 6.viii.2012, E. Helve (1, ZMUO), ZMUO.004794, KJ962308; Kuusamo, Aroniemi (66.2741° N, 29.7033° E), 14.vi.2014, Mikko Pentinsaari (2, ZMUO), ZMUO.016797, ZMUO.016798, COLFH362-14; COLFH363-14; GERMANY: Laucha an der Unstrut, C. Schenkling (4, CNCI); Bavaria, Holzkirchen, 19.v.1957–8.v.1959, K. Gaigl (3, ZSM); Bavaria, Dietramszell, 26.iv.1959, K. Gaigl (1, ZSM); POLAND: Owl Mountains (Eulengeb) (2, MIZ), COL000397; Sudety-G, Stolowe, Zacisze, 15.v.1947, M. Wegrzecki (1, MIZ), COL000398; Sierakow, 15.viii.1950, B. Burakowski (1, MIZ), COL000399; Schlesien (1, MIZ), COL000401; RUSSIA: 2011, B. Korotyeav (3, CMNC); SLOVAKIA: Čačín, Bystrická vrchovina hills (48° 40.3ʼ N, 19° 15.4ʼ E), 6.vii.2020, Michael Košťál (2, CMNC), LEW_95, OP215781 (GenBank CO1 accession number), OP218950 (GenBank ITS2 accession number), LEW_97, OP215782 (GenBank CO1 accession number), OP218951 (GenBank CO1 accession number). UNITED STATES OF AMERICA: Alaska: Moose Creek (64.71119° N, 147.10143° W), 26.v.–9.vi.2015, J. Hagelin, spruce, meadow, hanging Malaise (1, UAM), UAM100387793; Connecticut: Cornwall, 14.vii.1925, C.A. Frost (1, MCZC), MCZ-ENT00727088; Indiana: Morgan Co., 14.vii–7.viii.2012, K.E. Schnepp, Malaise Trap (1, CMNC), LEW_104, OP218952 (GenBank ITS2 accession number); Elkhart, Liebeck (3, MCZC), MCZ-ENT00726950, MCZ-ENT00726951, MCZ-ENT00700727186; Maine: Hampdan Highlands, 12.viii.1929, H.C. Fall Collection (1, MCZC), MCZ_ENT00727182; Cumberland Co., 7.v.1916, C.A. Frost Collection (2, MCZC), MCZ-ENT00726970, MCZ-ENT00727073; Massachusetts: Middlesex Co., Natick, 15.vii.1928, C.A. Frost (1, CNCI); Middlesex Co., Natick, 15.vii.1928–18.iv.1952, C.A. Frost, Salix catkins (1, CMNC; 3, MCZC), MCZ-ENT00726971 – MCZ-ENT00726973; Michigan: Cheboygan Co., Cheboygan, 12.v.1921, S. Moore (1, UAIC), UAIC1072870; “Mich.”, F.C. Bowditch Collection (1, MCZC), MCZ-ENT00726959; Missouri: St. Louis, 26.iii.1908–12.iv.1908, G.W. Bock (3, MCZC), MCZ-ENT00726949, MCZ-ENT00726977 – MCZ-ENT00726980; New Hampshire: Three Mile Island, 9.vi.1907–8.v.1927, Frederick Blanchard (3, MCZC), MCZ-ENT00727091, MCZ-ENT00727098, MCZ-ENT00727099; Mount Washington, 11.v.1910–16.vii.1930, H.C. Fall Collection, Liebeck Collection, F.C. Bowditch Collection and F. Waldo Dodge Collection (11, MCZC), MCZ-ENT00726944, MCZ-ENT00726948, MCZ-ENT00727080 – MCZ-ENT00727084, MCZ-ENT00727095, MCZ-ENT00726996, MCZ-ENT00727177, MCZ-ENT00727185; Mount Washington, 1874, Frederick Blanchard Collection (3, MCZC), MCZ-ENT00726974 – MCZ-ENT00726976; Farmington, 3.viii.1893–30.vii.1900, H.C. Fall Collection (2, MCZC), MCZ-ENT00700727183, MCZ-ENT00727184; “N.H.”, F.C. Bowditch Collection (4, MCZC), MCZ-ENT00726955 – MCZ-ENT00726958; New York: “N.Y.”, H.C. Fall (2, MCZC), MCZ-ENT00727180, MCZ-ENT00727181; Oregon: Burgess, A. Fenyes (1, CAS); Pennsylvania: Hazleton, Liebeck Collection (1, MCZC), MCZ-ENT00726984; Allegheny Co., Frederick Blanchard Collection and W.G. Dietz Collection (6, MCZC), MCZ-ENT00726967, MCZ-ENT00727094, MCZ-ENT00727100 – MCZ-ENT00727103; “Pa.”, H.C. Fall Collection, F.C. Bowditch Collection, and W.G. Dietz (6, MCZC), MCZ-ENT00726953, MCZ-ENT00726954, MCZ-ENT00726966, MCZ-ENT00727093, MCZ-ENT00727094, MCZ-ENT00727179.
Diagnosis. Length 2.5–2.9 mm. Cuticular colouration black and/or red in most specimens; rarely entirely pale yellow. Scales on pronotum uniform in colour (usually white) or only with central band of discolouration; lacking discoloured lateral bands. Metaventrite and metepisternum with scales relatively sparse, not obscuring underlying cuticle. Shiny carina running longitudinally along last ventrite of males (at least present as swelling at the middle of ventrite). Apex of aedeagus smoothly rounded (semicircular); not subquadrate or distinctly pointed. Internal sac tuberculate, with distinct sclerotised hook like structure; lacking any protruding basal structures. Distributed across temperate and boreal North America.
Natural history. This species has been collected on Populus tremuloides (Michaux) and Salix bebbiana Sargent in North America, and in Europe from S. capraea Linnaeus, S. viminalis Linnaeus, S. cinerea Linnaeus, and S. aurita Linnaeus (Hoffmann Reference Hoffmann1954). Specimens of E. bipunctatus have also been collected in Lindgren funnel traps set in the canopy of P. grandidentata Michaux in New Brunswick (Webster et al. Reference Webster, Anderson, Webster, Alderson, Hughes and Sweeney2016) and in fermenting bait traps (beer/wine and sugars) in Russia (Ruchin et al. Reference Ruchin, Egorov and Khapugin2021).
Distribution in North America. This species is distributed widely across Canada, from New Brunswick to the Northwest Territories, and is also known from several American states, including Alaska and Oregon, and east of the Great Plains.
Remarks. Molecular data indicate some level of differentiation between the European and North American populations of this species (see Figs. 1, 2); however, dissections and examinations of external morphology did not uncover any significant differences in morphology warranting formal naming. The limited CO1 and ITS2 data available (Figs. 1, 2) do not suggest North American and European populations mixing (i.e., introduction events), although broader sampling could be conducted to further investigate this possibility. Here, we treat these as the same species. This has implications for “E. borealis” (Carr, 1920), which is determined here to represent an all-red male E. bipunctatus on the basis of the following: (1) possessing a conspicuous, shiny carina extending longitudinally over the last ventrite (Fig. 3A), (2) with the metepisternum and lateral half of the metaventrite not densely punctate or scaled, and with the cuticle in those regions mostly visible (Fig. 3D), and (3) dissections of similar, all-red specimens from the Canadian Prairies are all E. bipunctatus. For this reason, E. borealis is hereafter treated as a junior synonym of E. bipunctatus. Label data for the holotype of E. borealis (examined) are as follows: CANADA, Alberta, Edmonton, 24.v.1919, F.S. Carr [Edmonton Alta 24.v.1919 F.S. Carr]. Type Elleschus borealis, No. 419. CNC 379701.
Ellescus californicus (Casey, 1885), resurrected status
Elleschus californicus Casey, 1885 (original description).
Elleschus ephippiatus var. californicus; O’Brien and Wibmer Reference O’Brien and Wibmer1982: 116 (catalogue, distribution).
Elleschus ephippiatus; Poole and Gentili Reference Poole and Gentili1996: 215 (catalogue).
Notes about the type series. This species was described from two specimens collected in California, United States of America. The holotype (USNM) is labelled with one brown rectangular label that reads “Cal.”, a second brown rectangular label that reads “californicus type. Casey.”, a third brown rectangular label that reads “CASEY bequest 1925”, a red rectangular label that reads “TYPE USNM 36721”, and an institutional ID label that reads “USNMENT 01448984”.
Specimens examined
Holotype. UNITED STATES OF AMERICA: California: Casey (1, USNM), CASEY bequest 1925, TYPE USNM 36721, USNMENT 01448984.
Other material. UNITED STATES OF AMERICA: California: Sutter Co., Nicolaus, 18.iv.1971, Fred G. Andrews (1, CMNC); San Bernardino Co., Needles, 8.iv.1989, W.F. Barr, on willow (2, CMNC); San Bernardino Co., Victorville, 16.v.1955, W.R.M. Mason (1, CNCI); San Bernardino, F.C. Bowditch Collection (3, MCZC), MCZ-ENT00727018, MCZ-ENT00727020, MCZ-ENT00727022; Kern Co., Wofford Heights, 14–15.vii.1992, Henry and Anne Howden (4, CMNC); Greeley, W.G. Dietz Collection (4, MCZC), MCZ-ENT00727048 – MCZ-ENT00727051; Kern. Co., Alta Sierra, 15.vii.1992, Henry and Anne Howden (3, CMNC); Modoc Co., Cedarville, 1.iv.1946, W.F. Barr (1, CMNC); Los Angeles Co., Boquette, Boquet Canyon, 11.iv.1974, M.W. Hetz (1, UAIC); Sonoma Co., 2 mi. N of Cloverdale, 27.iii.1964, C.W. O’Brien, on Salix (6, CNCI); Humboldt Co., Alton, 1.viii.1937, B.P. Bliven (6, CAS); Humboldt Co., Willow Creek, 13.vi.1916, F.E. Blaisdell (22, CAS); Humboldt Co., Myers, 22.ix.1935, B.P. Bliven (4, CAS); Oroville, 30.iv.1927–24.vi.1927, H.H. Keifer, on Salix hindsiana (2, CAS); Oroville, 21.iv. 1928, H.H. Keifer (1, CAS); Trinity Co., Carrville, 1.vi.1913, Van Dyke Collection (1, CAS); Monterey Co., Carmel, 5.viii.1917, L.S. Slevin Collection (1, CAS); Sacramento, 27.iii.1937, B.E. White (2, CAS); Ahwahnee, May (2, CAS; 1, MCZC), MCZ-ENT00727156; Castle Crag, vii, A. Fenyes (2, CAS); Kaweah, R. Hopping (1, CAS); Truckee, 7.vii.1927, E.P. Van Duzee (1, CAS); Calaveras Co., Mokell Hill, 11.vi.1921, F.E. Blaisdell (1, CAS); Napa Co., Butts Canyon, 13 km NW of Pope Valley, 17.iv.1982, T.W. Davies (21, CAS); Yosemite, 27.v.1931, B.E. White (1, CAS); Riverside, 5.iv.1990, H.C. Fall Collection and F. Blanchard Collection (8, MCZC), MCZ-ENT00727064 – MCZ-ENT00727067, MCZ-ENT00727145 – MCZ-ENT00727148; Ventura, 3.iv.1892, H.C. Fall Collection (1, MCZC), MCZ-ENT00727149; Lassen Co., 4.vi.1913, H.C. Fall Collection and C.A. Frost Collection (9, MCZC), MCZ-ENT00727076, MCZ-ENT00727077, MCZ-ENT00727150 – MCZ-ENT00727155; Plumas Co., 17.viii.1917, H.C. Fall Collection (2, MCZC), MCZ-ENT00727157, MCZ-ENT00727158; Castle Crags, vii, H.C. Fall Collection (1, MCZC), MCZ-ENT00727161; Visalia, Culbertson (1, MCZC), MCZ-ENT00727159; Lake Tahoe, 24.v.1879, W.G. Dietz Collection (1, MCZC), MCZ-ENT00727115; “Cal.”, Liebeck Collection (3, MCZC), MCZ-ENT00726986 – MCZ-ENT00726988; Oregon: Lake Co., 8 mi. W of Adel, 24.v.1973, K.J. Goeden (4, CMNC); Klamath Co., Sprague River canyon, 5 mi. E of Bly, 22.v.1958, Vertrees and Schuh, on Salix (1, CMNC).
Diagnosis. Length 2.1–2.6 mm. Scales on pronotum usually not uniform in colour, with discoloured central and lateral bands. Metaventrite and metepisternum with scales relatively sparse not obscuring underlying cuticle. Distinct swelling at the tip of last ventrite of males (no longitudinal carina at middle of ventrite; often reduced or absent in smaller specimens). Apex of aedeagus quadrate to rounded, dorsoventrally flattened, and coming to a distinct, sharp point with two prominent tufts of setae. Aedeagus heavily sclerotised along lateral edges. Internal sac with protruding basal structures. Restricted to California and southern Oregon.
Distribution in North America. This species is restricted to California and southern Oregon. The apparent lack of overlap in the ranges of E. californicus and E. ephippiatus is notable, and further sampling of both species in the region may reveal differences in host plant preferences.
Remarks. This species occupies a region known for high rates of animal and plant endemism (Harrison Reference Harrison2013), including several endemic Salix and Populus species (Little Reference Little1979). Ellescus californicus has been collected from Salix exigua var. hindsiana (Bentham) Dorn, a plant endemic to southwest Oregon, California, and Baja California (Mexico) (Little Reference Little1979). Although this species was previously treated as a junior synonym of E. ephippiatus (O’Brien and Wibmer Reference O’Brien and Wibmer1982; Poole and Gentili Reference Poole and Gentili1996), the previously undocumented differences in male genitalia and lack of overlap in geographic range with E. ephippiatus support the status of E. californicus as a distinct species. Although molecular data were not secured for E. californicus due to a lack of recently collected specimens in examined museum collections, this species is clearly closely related to E. ephippiatus as it possesses (1) a prominent swelling at the tip of the last ventrite (males only), (2) discoloured (scales) pronotal bands, and (3) a pointed aedeagus and internal sac with protruding basal structures.
Ellescus michaeli Lewis and Anderson, new species
https://zoobank.org/urn:lsid:zoobank.org:act:469C9178-DB98-443B-A23C-E65BA1B61FFB
Specimens examined
Holotype. CANADA: Ontario: Mer Bleue Bog, 45.3973° N, 75.5144° W, 13.v.2021, R.S. Anderson, male beaten off Salix sp., (male, CMNC).
Paratypes. CANADA: Alberta: Edmonton, 10.v.1915–27.v.1919, F.S. Carr (2, UAIC; 1 CNCI; 1 MCZC), MCZ-ENT00727116; Edmonton, Liebeck Collection (3, MCZC), MCZ-ENT00727009 – MCZ-ENT00727011; Peace River (18 mi. S), 2.vi.1950, P. Rubtsoff (3, CNCI); Ghost Dam, 10.vi.1980, B.F. and J.L. Carr (1, CNCI); Calgary, 21.v.1956, B.F. and J.L. Carr (1, CNCI); Manitoba: Aweme, 15–26.v.1903, N. Criddle (1, DEBU; 5, MCZC), DEBU01089099, MCZ-ENT00726992 – MCZ-ENT00726995, MCZ-ENT00727079; Riding Mountain National Park, 4–6.vi.1938, W.J. Brown (3, CNCI); Carberry, 8–10.v.1953, Brooks–Kelton (5, CNCI); Makinak (4, CNCI); Winnipeg, 24.v.1909–27.iv.1916, J.B. Wallis (7, RAM), 1983.001; Ontario: Prince Edward Co., 10.v.1915–26.v.1955, Brimley (11, CNCI); Smoky Falls, Mattagami River, 8.vi.1934, G.S. Walley (1, CNCI); Ottawa, 7.v.1960–11.vi.1965, A.T. Howden (17, CMNC); Kanata, Corkstown Rd., 11.v.1975, J.D. Read (1, CMNC); North Gower, 28.iv.–4.v.1987, L. LeSage, old beaver meadow, attracted with allylisothiocyanate (2, CMNC); Ottawa, Mer Bleue Bog (45.3973° N, 75.5144° W), 13.v.2021, R.S. Anderson, beaten off Salix sp. (15, CMNC), LEW_145, OP215794 (GenBank CO1 accession number), OP218955 (GenBank ITS2 accession number), LEW_146, OP215795 (GenBank CO1 accession number), LEW_147, OP218956 (GenBank ITS2 accession number), LEW_148, OP215796 (GenBank CO1 accession number), OP218957 (GenBank ITS2 accession number), LEW_149, OP215797 (GenBank CO1 accession number); Mer Bleue, 10.v.1933, W.J. Brown (75, CNCI); Wellington Co., Guelph, 3.v.1982, D. Morris (1, DEBU), DEBU01089097; Marmora, 22.iv.–8.viii.1952, J.C. Mitchell, on Salix (6, CNCI); Quebec: Cantley, 45.591637° N, 75.785395° W, 23.iv.2021, J.H. Lewis, beaten off Salix sp., (1, CMNC), LEW_130, OP215793 (GenBank CO1 accession number); Rigaud (4, MCZC), MCZ-ENT00727124 – MCZ-ENT00727127; Montreal, v.1929–10.v.1931, H.C. Fall Collection and Liebeck Collection (2, MCZC), MCZ-ENT00727007, MCZ-ENT00727160; Aylmer (Gatineau), Pink Road (near CMN natural history campus), 21.iv.2008, L. LeSage, on Salix discolor catkins (8, CNCI); Cantley, dune on hill near chemin Holmes (45.591708° N, 75.784886° W), 22.v.2020, J.H. Lewis, on female Salix catkins (2, CMNC), LEW_67, OP215790 (GenBank CO1 accession number), OP765914 (GenBank ITS2 accession number), LEW_68, OP215791 (GenBank CO1 accession number), OP218942 (GenBank ITS2 accession number); Gatineau, Mont Cascades (45.590976° N, 75.850493° W), 14.v.2021, J.H. Lewis, beaten from Salix (1, CMNC); Aylmer, disturbed lot across road from 1740 Pink Road (45.441961° N, 75.811547° W), 10.v.2020, J.H. Lewis, on female Salix catkins (1, CMNC), LEW_70, OP215792 (GenBank CO1 accession number); Aylmer, disturbed lot across road from 1740 Pink Road (45.441961° N, 75.811547° W), 6.v.2022, J.H. Lewis, on Salix catkins (20, JHLC); Gatineau, Boucher Forest (45.416327° N, 75.826699° W), 18.v.2021, J.H. Lewis, beaten from Salix (3, CMNC); 28.vi.1965, Malaise trap (1, CNCI); Kazubazua, 25.v.1933, W.J. Brown (1, CNCI); Mont St. Hilaire, v.1909 (1, CNCI); Saskatchewan: Saskatoon, 21.v.1940, A.R. Brooks (1, CNCI); Canora, 28.v.1954, Brooks–Wallis (1, CNCI); Fish Creek, 20.v.1921, K.M. King (1, CNCI); UNITED STATES OF AMERICA: Maine: Penobscot Co., Orono, 24.v.1948 (2, UAIC), UAIC1072936, UAIC1072937; Michigan: Oakland Co., Royal Oak, 10.vi.1916, A.W. Andrews (2, MCZC), MCZ-ENT00727163, MCZ-ENT00727164; Whitefish Point, 26.vii.1914, H.C. Fall Collection (1, MCZC), MCZ-ENT00727162; Lansing, Liebeck Collection (1, MCZC), MCZ-ENT00727015; Minnesota: Otter Tail Co., Maplewood St. Pk., 28.v.1983, I.S. Askevold, sweeps of Salix (2, CMNC; 1, UAIC); New Hampshire: Mount Washington, vii.1910, Liebeck Collection (1, MCZC), MCZ-ENT00727001; Pennsylvania: Warren, A. Fenyes (1, CAS); Wisconsin: University of Wisconsin–Madison Arboretum, 30.iv.1949, R.H. Jones (7, CNCI).
Diagnosis. Length 1.9–2.2mm. Scales on pronotum usually not uniform in colour, with discoloured central and lateral bands. Metaventrite and metepisternum with scales relatively sparse not obscuring underlying cuticle. Metaventrite (black to dark red) usually not contrasting with femora, tibiae, or latter half of rostrum. Elytral colouration distinct; largely dark with contrasting orange band in apical half. Lacking a distinct swelling at the tip of last ventrite of males. Apex of aedeagus coming to a broad, rounded point, with apical edges flattened laterally; also with setae distributed along the surface. Internal sac with protruding basal structures. Distributed across temperate North America.
Distribution in North America. This species is broadly distributed across Canada, from Quebec to Alberta, and is also known from several northern American states including Maine, Minnesota, and Wisconsin. The species has been collected in large series from the Ottawa–Gatineau region, Ontario and Quebec, where it is often the most abundant species of Ellescus; it is only known from short series or single specimens in most other provinces and states.
Variation. Colour is relatively conserved in this species across its range (overall dark, with a bright, contrasting orange band across the apical half of the elytra; Fig. 8A, B). However, all-red specimens are known from Otter Tail Co., Minnesota and two localities in Manitoba (e.g., Fig. 8C, D). All-red forms also occur in E. ephippiatus and E. bipunctatus and are usually cases of geographic variation rather than teneral or aberrant colouration. The all-red forms of Ellescus michaeli may also represent instances of geographic variation. All-red forms are especially common in western Canadian population of Ellescus, and it is not uncommon to encounter series which contain mixtures of all-red forms of all three species. Dissection of males and to a lesser extent females is sometimes necessary in those cases to accurately identify specimens to the species level.
Etymology. This patronym is dedicated to Dr. Michael Košťál (Slovakia), a weevil expert who graciously collected and mailed fresh specimens of Palearctic Ellescus for use in this project.
Natural history. This species has been collected from S. discolor Muhlenberg, S. petiolaris Sm., and S. bebbiana Sargent. Collections for this study revealed that this species emerges in early spring and can be found inactive on female Salix catkins on colder mid- to late April days. This species appears to be active earlier in the spring than E. ephippiatus, and unlike that species, Ellescus michaeli has yet to be collected from Populus trees.
Remarks. The combination of unique male genitalia, colour, and ITS2 sequence data (Fig. 2) support E. michaeli as a new species. Notably, CO1 did not delineate E. michaeli and E. ephippiatus (see Fig. 1), but ITS2 reliably did so for all specimens examined. This illustrates the importance of using multiple markers in taxonomy and also that CO1 alone does not separate species in all cases. Molecular data (CO1, ITS2) and morphology indicate that E. michaeli is closely related to the more widespread and variable E. ephippiatus. To preclude a possible introduction, all currently recognised Old World Ellescus were examined and are all morphologically unlike E. michaeli.
Ellescus ephippiatus (Say, 1832)
Alyca ephippiata LeConte, 1876 (description).
Elleschus ephippiatus; Blatchley and Leng Reference Blatchley and Leng1916: 284 (identification; distribution); Hatch Reference Hatch1971: 342 (identification; catalogue); O’Brien and Wibmer Reference O’Brien and Wibmer1982: 116 (catalogue, distribution).
Ellescus ephippiatus; Hight Reference Hight1988: 276 (apparent alternate host use; dubious); Ciegler Reference Ciegler2010: 71 (identification, distribution); Prena Reference Prena2018: 382 (taxonomic comments, syntype location); Bousquet et al. Reference Bousquet, Bouchard, Davies and Sikes2013: 326 (catalogue, distribution); Webster et al. Reference Webster, Anderson, Webster, Alderson, Hughes and Sweeney2016: 374 (distribution).
Eririhinus ephippiatus Say, 1832 (original description)
Eririhinus rufous Say, 1832 (description)
Eririhinus rufus; O’Brien and Wibmer Reference O’Brien and Wibmer1982: 116 (catalogue, distribution)
Notes about the type series. This species was described from an unknown number of specimens collected in Indiana, United States of America. Here, we adopt a definition of E. ephippiatus that is based on a syntype specimen deposited in the Swedish Museum of Natural History (NHRS) Schönherr collection (NHRS-JLKB000073656), the type locality, and the original description. See Remarks.
Specimens examined
Syntype. UNITED STATES OF AMERICA: Indiana (see Remarks), (1, NHRS), NHRS-JLKB000073656.
Other material. CANADA: Alberta: Calgary, 31.vi.1957–19.v.1978, B.F. and J.L. Carr (3, CNCI); Elbow Falls, 13.vi.1959, B.F. and J.L. Carr (5, CNCI); Exshaw, 27.vi.1954, B.F. and J.L. Carr (1, CNCI); Fort Macleod, 20.vi.1976–23.vii.1977, B.F. and J.L. Carr (3, CNCI); Fitzgerald, 15.vi.1988, B.F. and J.L. Carr (1, CNCI); Lethbridge, 24.vi.1956, O. Peck (1, CNCI); Lethbridge, Oldman River, 22.vi.1956, E.E. Sterns (3, CNCI); Lethbridge, 6.v–30.vi.1930, J.H. Pepper (9, CNCI); Writing-on-Stone Provincial Park (25 mi. E), 15.vii.1980, G. Gibson, sweeps (2, CMNC); Writing-on-Stone Provincial Park (0.5 mi. E), 14.vi.1982, R.S. Anderson and G.A.P. Gibson, sweeping (6, CMNC); Sandy Point campground, 32 km S of Empress, 2.viii.1981, R.S. Anderson (2, CMNC); Medicine Hat, Kin Coulee Park, 13.vi.1982, R.S. Anderson and G.A.P Gibson (13, CMNC); Medicine Hat, 3.vi.1920–vi.1930, F.S. Carr (3, CNCI); Medicine Hat, 17.vi.1930, J.H. Pepper (1, CNCI); Medicine Hat, 21.vii.1962–5.viii.1979, B.F. and J.L. Carr (8, CNCI); Manyberries, 30.vi.1930, J.H. Pepper (2, CNCI); McMurray, 4.vi.1953, W.J. Brown, on Salix (6, CNCI); Edmonton, 27.v.1985, B.F. and J.L. Carr (1, CNCI), CNC COLEO DNA 00157038 (BOLD sample ID); Edmonton, 15.v.1915–26.iv.1922, F.S. Carr (3, MCZC), MCZ-ENT00727086, MCZ-ENT00727188, MCZ-ENT00727189; British Columbia: Oliver (5–7 mi. N), 21.v.–2.vi.1958, H. and A. Howden (2, CNCI); Oliver, 15.v.1959, L.A. Kelton (11, CNCI); Manitoba: Churchill, 10.vii.2008, S. McCubbin and J. Sones (1, CBG), CHU-MXC-090, JSCOC148-09; Churchill, 13–17.vi.1952, J.G. Chillcott (2, CNCI); Aweme, 28.vi.1920, N. Criddle (1, CNCI); Aweme, 24.v.1912, H.C. Fall Collection (2, MCZC), MCZ-ENT00727196, MCZ-ENT00727197; Glenboro, 9.vi.1920, S. Criddle (1, CNCI); Eastman Region, Victoria Beach, 6.vii.1985, I.S. Askevold (2, CMNC); Eastman Region, Brokenhead, road crossing at Hwy #15, 20 km E of Anola, I.S. Askevold and D.A. Pollock (4, CMNC); Wapusk National Park (58.7231° N, 93.4597° W), 15.vi–22.vi.2014, D. Iles (1, CBG), BIOUG17638-A05, CNWAA700-14; Gillam, 18.vi–1.vii.1950, W.J. Brown (3, CNCI); Carberry, 20.v.1953, Brooks–Kelton (4, CNCI); Riding Mountain National Park, 5–11.vi.1938, W.J. Brown (2, CNCI); Warkworth Creek (near Churchill), 21.vi.1952, J.G. Chillcott (6, CNCI); New Brunswick: Sunbury Co., Maugerville (off Route 105 (45.8662° N, 66.4559° W), 4.vi.2014, R.P. Webster, flood plain forest, sweeping roadside foliage (1, RWC); Northwest Territories: Yellowknife, 29.v.1953, J.G. Chillcott (2, CNCI); Yellowknife, 24.vi.1949, R.R. Hall (1, CNCI); Ontario: Prince Edward Co., 19.viii.1923–17.v.1950, J.F. Brimley (15, CNCI); Rouge National Urban Park, west of Glen Rouge campground (43.8040° N, 79.1455° W), 7.vi.2013 (1, CBG), BIOUG13533-G06, SSROB2985-14; Kitchener, Huron Heights Secondary School (43.3924° N, 80.4663° W), 22.iv.–3.v.2013, Chris Roth (1, CBG), BIOUG05652-F10, SMTPB14415-13; Bruce Co., Inverhuron Provincial Park (front dunes) (44° 17ʼ 33ʼʼ N, 81° 35ʼ 28ʼʼ W), 15.vi.2003, M. Buck (1, DEBU), DEBU01127408; Huron Co., Goderich, 22.vi.1977, K. Barber (1, DEBU), DEBU01089105; Wellington Co., Fergus, 5.iv.1997, S.A. Marshall (1, DEBU), DEBU01068482; Middlesex Co., Ailsa Craig, 22–22.vi.1983, E. Zaborski, flight intercept trap in turnip field (1, DEBU), DEBU01089107; Northumberland Co., Presqu’ile Provincial Park, Stonehedge Cottage, 16.vi.2003, P.D. Careless (1, DEBU), DEBU01089106; Wellington Co., Guelph, 10.vii.1978, K.N. Barber, reared from Populus tremuloides cotton balls (9, DEBU), DEBU01089088-DEBU01089096; Kenora District, 14 mi. S of Pickle Lake, 20.vi.1973, Henry and Anne Howden (1, CMNC); Wellington Co., Erin, 17.vi.1979, Jessica Ernst (1, CMNC); Quebec: Nord-du-Québec, James Bay, Long Point (53.9752° N, 79.0685° W), 7.vii.2018, Pierre de Tonnacour, beaten from Salix brachycarpa var. brachycarpa (6, CMNC; 2, CNCI); Kazubazua, 25.v.1933, W.J. Brown (7, CNCI); Saskatchewan: Elbow, 23.vi.1954, Brooks–Kelton (7, CNCI); Saskatoon, 3.vi.1949, J.R. Vockeroth (3, CNCI); Saskatchewan Landing Provincial Park (50° 39ʼ N, 107° 56ʼ W), 25.v.1955, J.R. Vockeroth (8, CNCI); Hatton, 11.vi.1929, K.M. King and R. Glen (5, CNCI); Township 6, Region 28, 23.vi.1990, B.F. and J.L. Carr (1, CNCI), CNC COLEO DNA 00157037 (BOLD sample ID); Township 8, Region 29, 23.vi.1990, B.F. and J.L. Carr (1, CNCI), CNC COLEO DNA 00157036 (BOLD sample ID); Yukon: Fort Yukon, 2.vii.1924, H.C. Fall Collection (2, MCZC), MCZ-ENT00727194, MCZ-ENT00727195; Dawson, 16.vi.1916–26.vi.1924, H.C. Fall Collection (4, MCZC), MCZ-ENT00727190 – MCZ-ENT00727193; Tatchun Creek, 25.vi.1979, B.F. and J.L. Carr (2, CNCI), CNC COLEO DNA 00157039 (BOLD sample ID); Mayo, 5.vii.1955, B.F. and J.L. Carr (5, CNCI); Willow Creek, at kilometre 621 along Klondike Highway, 5.vi.1980, R.J. Cannings (2, CMNC); McQuesten (10 km E), 1.vi.1980, R.J. Cannings (1, CMNC); Ross River, 9 km S on Campbell Highway (61° 54ʼ N, 132° 25ʼ W), 12.vi.1981, C. Guppy (2, CMNC); Ross River (132° 30ʼ N, 61° 56ʼ W), 20–22.vi.1960, J.E.H. Martin and E.W. Rockburne (5, CNCI); Stewart Crossing (18 km W), 2.vi.1980, R.J. Cannings (2, CMNC); Hwy 2, 22 mi. N of Hwy 1, 25.vi.1979, B.F. and J.L. Carr (2, CNCI), CNC COLEO DNA 00157040 (BOLD sample ID); Kirkman Creek, 13.vi.1928, A.C. Davis (36, CNCI); Swim Lakes (133° 00ʼ N, 62° 13ʼ W), 15.vi.1960, J.E.H. Martin (1, CNCI); UNITED STATES OF AMERICA: Alabama: Monroe Co., Haines Island Park (31° 43ʼ 23ʼʼ N, 87° 28ʼ 10ʼʼ W), 26–31.v.1995, T.L. Schiefer, mercury vapour and blacklight trap (3, MEM); Macon Co., 4 mi. E Shorter along I-85, 16.iv.1984, D.A. Rider (1, LSAM); LSAM0144883, LSAM0144885; Mobile, 2.iv.1911, H.P. Loding, on willow (4, MCZC), MCZ-ENT00726996 – MCZ-ENT00726999; Alaska: Fort Yukon, 2.vii.1924, H.C. Fall Collection (2, MCZC), MCZ-ENT00727195, MCZ-ENT00727195; Eagle, 18.vi.1928 (10, CNCI); Washington Creek, 21.vi.1977, J. Cowling (1, UAM), UAM100008612; Selawik National Wildlife Refuge (66.85873° N, 158.16618° W), 24.vi.2010, D.S. Sikes, open sand dunes sweep (11, UAM), UAM100283227, UAM100283242, UAM100283250, UAM100283254, UAM100283256, UAM100283481 (LEW_78; OP215787 (GenBank CO1 accession number), OP218946 (GenBank ITS2 accession number)), UAM100283503, UAM100283520 (LEW_79; OP215788 (GenBank CO1 accession number), OP218947 (GenBank ITS2 accession number)), UAM100283521, UAM100283568, UAM100283596; Nogahabara Dunes (Koyukuk National Wildlife Refuge) (65.65846° N, 157.49608° W), 13.vi.2010, J.A. Slowik, willow sweep (1, UAM), UAM100327118, LEW_77, OP215786 (GenBank CO1 accession number), OP765915 (GenBank ITS2 accession number); Arizona: Apache Lake, 10.v.1932, H.C. Fall Collection (1, MCZC), MCZ-ENT00727174; Santa Cruz Co., 4.iv.1937, R.A. Flock (2, UAIC), UAIC1072923; Coconino Co., Oak Creek, Chavez King south of Sedona, 14.vi.1978, M.W. Sanderson (1, UAIC), UAIC1072941; Graham Co., Aravaipa Canyon, 24.viii.1976, D.S. Chandler, sift oak litter (1, UAIC), UAIC1072925; Pima Co., Tucson, “Apr, 191” (2, UAIC; 4, MCZC), UAIC1072924, UAIC1072939, MCZ-ENT00727170 – MCZ-ENT00727173; Yavapai, Co., Oak Creek, Baldwin’s King, 9.vi.1978, M.W. Sanderson (1, UAIC), UAIC1072940; Yavapai Co., Dead Horse Ranch State Park, Verde River near bridge (34.7502° N, 112.0217° W), 5.vi.2011 (1, CBG), BIOUG02273-E11, BBCCA2871-12; Maricopa Co., 4 mi. E of Buckeye along Gila River, 1.v.2018, Kyle E. Schnepp, beaten from willow (Salix sp.) (3, CMNC); Maricopa Co., Pheonix, 3.iv.1942, P.C. Grassman (3, UAIC), UAIC1072926; Maricopa Co., Pheonix, 9.iii.1941, T. Parker (7, UAIC), UAIC1072928-UAIC1072934; Javapaj Co., Cottonwood, 24–25.vi.1978, C.V. Nidek (3, CMNC); “Ariz” (2, DEBU), DEBU01089100, DEBU01089102; Arkansas: Knobel, viii., Liebeck Collection (2, MCZC), MCZ-ENT00727002, MCZ-ENT00727003; Washington Co., 2 mi. SW of West Fork, 12.v.1991, C. Carleton (1, LSAM), LSAM0210499; Washington Co., Devil’s Den State Park, 1–2.vii.1984, E.G. and M.A. Riley (1, LSAM), LSAM0143750; Toad Suck Park, campground transition between park and forest (35.0724° N, 92.5449° W), 2.vii.2011 (1, CBG), BIOUG02327-D08, BBCCA4766-12; Colorado: Boulder, 22.v.1907, S.A. Rohwer (1, MCZC), MCZ-ENT00727143; La Veta Pass (13 mi. W), 12.vi.1982, B.F. and J.L. Carr (1, CNCI); Chaffee Co., O’Hayer Lake, 22.vi.1983, I.S. Askevold (1, UAIC); Las Animas, 15–16.vi.1982, B.F. and J.L. Carr (5, CNCI); Pueblo Co., Colorado City, 9.vi.1982, B.F. and J.L. Carr (1, CNCI); Pueblo Co., 18.vi.1957, G.H. Nelson, beating Salix (2, MCZC), MCZ-ENT00727097; “Col.”, Liebeck Collection and W.G. Dietz Collection (3, MCZC), MCZ-ENT00727012, MCZ-ENT00727042, MCZ-ENT00727043; Garland, F.C. Bowditch Collection (1, MCZC), MCZ-ENT00727030; Florida: DeSoto Co., Fort Ogden, 8.iv.1952, J.R. Vockeroth (4, CNCI); Seminole Co., Sanford, 19.iv.1927, A.C. Davis (1, CNCI); Liberty Co., Apalachicola River, Hwy 20 near Bristol (30° 26.2ʼ N, 85° 00.0ʼ W), 17.iv.2010, S.M. Clark (2, UAIC); Leon Co., Tall Timbers Res. Station, Sheep Island, 17.iv.1978, G.J. Wibmer (1, UAIC); Leon Co., Tallahassee, 3.iv.1976, G.B. Marshall, on fruiting Salix (2, CMNC; 60, UAIC); Alachua Co., Archer (2 mi. W), 23.iii.1953, Henry F. Howden (1, CMNC); Alachua Co., Gainesville NATL, 23.iii.2016, Kyle E. Schnepp, beaten from Salix (5, CMNC), LEW_74, OP215785 (GenBank CO1 accession number), OP218945 (GenBank ITS2 accession number); Glades Co., Palmdale, 31.iii.2018, Kyle E. Schnepp, swept from vegetation (1, CMNC); Miami–Dade Co., Paradise Key, 6.iv.1951, Henry and Anne Howden (1, CMNC); Volusia Co., South Daytona, 17.iv.1959, “S.F.B.” (1, CMNC); Miami–Dade Co., Chekika Street (recreation area), 50 km SW of Miami, 1.xi.1984–3.xii.1985, Stewart and Jarmila Peck, “Grossman HammockFor. Malaise-FIT” (1, CMNC); Georgia: Savannah, 27.v.1926, H.C. Fall Collection (5, MCZC), MCZ00727165 – MCZ00727169; “Geo.”, F.C. Bowditch Collection (3, MCZC), MCZ-ENT00727035 – MCZ-ENT00727037; Idaho: Cassia Co., Burley, 2.vi.1986, B.F. and J.L. Carr (1, CNCI); Nez Perce Co., Lewiston, 22.v.1986, B.F. and J.L. Carr (2, CNCI); Cincinnati, 17.vi.1906, C.A. Frost Collection (1, MCZC), MCZ-ENT00727078; Illinois: Union Co., Dongola (Hogam Bottoms), 19.vi–4.ix.1968, D. Dillow, maple/cypress/beech buttress (6, LSAM) LSAM0210492 – LSAM0210497; Coles Co., Charleston, 12.v.1951, D.M. Tuttle Collection (39, UAIC), UAIC1072875, UAIC1072878-UAIC1072898, UAIC1072905-UAIC1072914, UAIC1072916-UAIC1072921, UAIC1072935; “Ill.”, Frederick Blanchard Collection (1, MCZC), MCZ-ENT00727062; Indiana: Jackson Co., Muscatatuck N.W.R., 26.iv.2013, Gareth S. Powell (1, CMNC); Jackson Co., Muscatatuck N.W.R., 10.v.–7.vi.2013, Kyle E. Schnepp, Lindgren funnel trap (1, CMNC); Iowa: Clinton Co., DeWitt, 21.v.1928, G.S. Walley (1, CNCI); Ames, 1.vi.1931, G. Hopping (2, CAS); Kansas: Riley Co., 20.iv.1958–9.v.1960, J.R. McCoy Collection (7, MEM); Logan Co., State Lake, 7.vi.1964, C.W. O’Brien (5, UAIC; 6, CAS); Douglas Co., F.H. Snow (4, CNCI); Douglas Co., Lawrence, iv.1930–23.iv.1962, L.W. Brown and W.J. Brown (13, CNCI); Reno Co., Medora, 2.v.1962, W.J. Brown (2, CNCI); Stockton, 7.vii.1939, J.W. Green (1, CAS); W. Knaus (1, CAS); “Kan.”, F.C. Bowditch Collection, W.G. Dietz Collection, Frederick Allen Eddy (1, MCZC), MCZ-ENT00727029, MCZ-ENT00727038 – MCZ-ENT00727041, MCZ-ENT00727106 – MCZ-ENT00727113; Louisiana: Harahan, 26.vii.1944, F.G. Werner (1, MCZC), MCZ-ENT00727120; Tallulah, 22.vi.1933, C.A. Frost Collection (2, MCZC), MCZ-ENT00727074; East Baton Rouge Parish, Baton Rouge, Mississippi River Levee, 21.iii.2003, M.G. Radtke, sweepnet (1, LSAM); East Baton Rouge Parish, Louisiana State University campus (30° 24.5ʼ N, 91° 10.5ʼ W), 22–23.v.2010, J.S. Park (1, LSAM), LSAM0201337, MDM2010; East Baton Rouge Parish, Baton Rouge, 12.v.1986, E.G. Riley (1, LSAM); East Baton Rouge Parish, Baton Rouge, 26.iv.1986, E.G. Riley, flight trap (1, LSAM), LSAM0143498; East Baton Rouge Parish, Baton Roufe GSRI, 1.iv.1991, V.L. Moseley, mating on willow fruit (9, LSAM), LSAM0145660-LSAM0145664, LSAM0145673, LSAM0145675, LSAM0145679, LSAM0145681; East Baton Rouge Parish, Baton Rouge, 8.v.1988–17.v.1989, D.A. Rider, mercury vapour and blacklight (6, LSAM), LSAM0143031, LSAM0143038, LSAM0146124, LSAM0146127, LSAM0146137, LSAM0146167; East Baton Rouge Parish, Baton Rouge, 28.v.–3.vi.1986, C.B. Barr and E.G. Riley, yellow sticky board trap in cypress tree (3, LSAM), LSAM0142726-LSAM0142728; East Baton Rouge Parish, Louisiana State University Campus, 16.v.1984–15.vii.1985, D.A. Rider (17, LSAM), LSAM0143151, LSAM0143173, LSAM0143175, LSAM0143177, LSAM0143197, LSAM0143200, LSAM0143203, LSAM0143315, LSAM0143319, LSAM0143357, LSAM0143358, LSAM0143816, LSAM0144843, LSAM014497, LSAM0145201, LSAM0145277, LSAM0145281; Jefferson Parish, Harahan, iv.1944, F.G. Werner (1, UAIC), UAIC1072871; Jefferson Parish, Jean Lafitte National Park, 8.v.1983, C.B. Barr (1, LSAM), LSAM0145397; East Baton Rouge Parish, “E of LA 3025, 1.2 mi. S. Central”, 27.iv.–12.v.1985, C.B. Barr, mercury vapour and black light (3, LSAM), LSAM0144680, LSAM0145456, LSAM0145457; St. Tammany Parish, I-10 at Pearl River, 17.vi.1982, C.B. Barr and E.G. Riley, black light (1, LSAM), LSAM0145329; Tangipahoa Parish, Tangipahoa River between Arcola and Tickfaw, 12.iv.1986, C.B. Barr (1, LSAM), LSAM0144655; Orleans Parish, New Orleans, 3.vii.1969, light trap (1, MEM); Orleans Parish, New Orleans, LA Nature Center, 25.iv.1984, C.B. Barr, black light trap (2, LSAM), LSAM0144780, LSAM0144782; Vermillion Parish, Pecan Island, 11.iii.1982, C.B. Barr (1, LSAM), LSAM0145323; Vermillion Parish, White Lake Research Station (29° 55.141ʼ N, 92° 30.830ʼ W), 20.iii.2009, M. Gimmel, UV light (1, LSAM), LEW_109, OP215789 (GenBank CO1 accession number), OP218953 (GenBank ITS2 accession number); West Feliciana Parish, Feliciana Preserve (30° 47ʼ N, 91° 15ʼ W), 14.iv.2003, A. Tishechkin, beating low vegetation (1, LSAM); Corney Lake, 25–28.vi.1983, B.F. and J.L. Carr (6, CNCI); Maryland: Easton, 22.vi.1918, J.G. Sanders (2, MCZC), MCZ-ENT00727199, MCZ-ENT00727200; Massachusetts: Lowell, H.C. Fall Collection and Frederick Blanchard Collection (8, MCZC), MCZ-ENT00727137, MCZ-ENT00727138, MCZ-ENT00727057 – MCZ-ENT00727061; Milton, 17.v.1903, P.G. Bolster Collection (1, MCZC), MCZ-ENT00727052; Michigan: Lansing, Liebeck Collection (2, MCZC), MCZ-ENT00727016, MCZ-ENT00727017; Mississippi: Attala Co., 1 mile east McCool (33° 12ʼ 46ʼʼ N, 89° 23ʼ 21ʼʼ W), 23.v.2005, J.A. MacGown and J.G. Hill, Berlese – soil and litter in mixed forest (1, MEM); Claiborne Co., Natchez Trace Parkway, Grindstone Ford (32° 00ʼ 10ʼʼ N, 90° 53ʼ 41ʼʼ W), 7.iii.2005, J.G. Hill, Berlese litter (1, MEM); Harrison Co., Gulfport C.T. Nature Ar. (30° 22ʼ 55ʼʼ N, 89° 03ʼ 23ʼʼ W), 9.v.2000, T.L. Schiefer, blacklight trap (1, MEM); Oktibbeha Co., 3 mi. W of Adaton (33° 29ʼ 00ʼʼ N, 88° 58ʼ 13ʼʼ W), 26.iii.2000, T.L. Schiefer, on flowering Prunus serotine (1, MEM); Oktibbeha Co., near Noxubee National Wildlife Refuge (33° 18ʼ 03ʼʼ N, 88° 46ʼ 08ʼʼ W), 3.iv.1998, T.L. Schiefer, on flowering Salix nigra (2, MEM); Oktibbeha Co., 8.5 mi. S Starkville (33° 19ʼ 58ʼʼ N, 88° 49ʼ 05ʼʼ W), 20.iv.1995, T.L. Schiefer, beating recently cut oaks (1, MEM); Oktibbeha Co., Starkville, MSU north of North Farm, 21.iv.1994, D.M. Pollock, sweeping (1, MEM); Oktibbeha Co., Starkville, 13.iii.1985, W.P. Chan (1, MEM); Oktibbeha Co., Noxubee Refugee, 5.ii.1982, G.L. Snodgrass, Berlese – litter hardwood forest (1, MEM); Oktibbeha Co., Noxubee National Wildlife Refuge (33° 24ʼ 11ʼʼ N, 88° 53ʼ 33ʼʼ W), 6.x.2008, J. MacGown and J.G. Hill, litter mixed pine hardwood forest (1, CMNC); Oktibbeha Co., 4 mi. E of Mississippi State University (33° 23ʼ 04ʼʼ N, 88° 46ʼ 02ʼʼ W), 23.ii.2014, J.L. Gesell (1, MEM); Issaquena Co., Shipland W.M.A. (32° 45ʼ 15ʼʼ N, 91° 07ʼ 44ʼʼ W), 3.viii.2005, J.A. MacGown and J.G. Hill, in leaf litter in floodplain hardwood forest (4, MEM); Jackson Co., Ocean Springs, 13.v.1984, Paul K. Lago (1, CMNC); Lafayette Co., Oxford, 4.vi.1982–30.vi.1983, Paul K. Lago (2, CMNC); Oktibbeha Co., Sessums (33° 23ʼ 31ʼʼ N, 88° 42ʼ 40ʼʼ W), 22.iv.–28.iv.1998, J.A. MacGown, Malaise trap (47, MEM); Sharkey Co., Sunflower WMA (32° 50ʼ 55ʼʼ N, 90° 48ʼ 24ʼʼ W), 21.vii.2003, R.L. Brown, Berlese trap – bottomland hardwood litter (2, MEM); Noxubee Co., Noxubee National Wildlife Refuge (33° 16ʼ 44ʼʼ N, 88° 46ʼ 39ʼʼ W), 8.vi.1998, T.L. Schiefer (8, MEM); Noxubee Co., Noxubee National Wildlife Refuge (33° 15ʼ 04ʼʼ N, 88° 44ʼ 51ʼʼ W), 31.vii.2008, J.A. MacGown, litter in pine–hardwood forest (2, MEM); Lee Co., Tombigbee State Park, 7.vi.1995, T.L. Schiefer, beating trees and shrubs (1, MEM); Smith Co., 1 mile North of Raleigh, 14–15.vi.1985, R. Brown and G. Burrows (1, MEM); Tate Co., Senatobia Wetlands (34° 37ʼ 05ʼʼ N, 89° 56ʼ 50ʼʼ W), 31.v.2018, R.J. Whitehouse, sweeping grasses and plants along water edge and field (1, MEM); Winston Co., Tombigbee National Forest (33° 11ʼ 50ʼʼ N, 89° 03ʼ 20ʼʼ W), 5.iv.1999, T.L. Schiefer, blacklight trap in mixed mesic forest (1, MEM); Missouri: St. Louis, M. Schuster (1, DEBU), DEBU01089103; Kansas City, Liebeck Collection (4, MCZC), MCZ-ENT00726989 – MCZ-ENT00726991, MCZ-ENT00727000; St. Louis, Liebeck Collection (3, MCZC), MCZ-ENT00727004 – MCZ-ENT00727006; “Mo.”, F.C. Bowditch Collection (1, MCZC), MCZ-ENT00727027; Montana: Deer Lodge Co., Anaconda, 4.vii.1962, J.B. Karren, on Salix (1, UAIC); Fergus Co., Little Box Elder Creek (by Hwy 19), 13.viii.1990, B.F. and J.L. Carr (1, CNCI); Kalispell, F.C. Bowditch (2, MCZC), MCZ-ENT00727033, MCZ-ENT00727034; Nebraska: Thomas Co., Halsey, 29–31.vii.1973, Henry and Anne Howden (1, CMNC); W. Knaus, A. Fenyes Collection (3, CAS); “Nebr.”, H.C. Fall Collection (4, MCZC), MCZ-ENT00727139 – MCZ-ENT00727142; Nevada: Elko Co., North Fork, Humboldt River at Elburz, 22.vi.1995, R.W. Baumann (1, UAIC); McCook, R. Hopping Collection (3, CAS); New Jersey: Phillipsburg, 8.viii.1909–12.vi.1931, J.W. Green (17, CAS); Camden, Liebeck Collection (1, MCZC), MCZ-ENT00727013; Newark, F.C. Bowditch Collection (1, MCZC), MCZ-ENT00727028; New Mexico: Jemez Mountains, v.1913, Jno. Woodgate (13, CAS); “N.M.”, W.G. Dietz (2, MCZC), MCZ-ENT00727045, MCZ-ENT00727046; New York: Staten Island, 6.vi.1986, Van Dyke Collection (53, CAS); North Carolina: Southern Pines, 8.vi.1911, A.H. Manee (1, MCZC), MCZ-ENT00727104; Oklahoma: Pushmataha Co., Clayton, 21.vi.1983, B.F. and J.L. Carr (1, CNCI); Cimarron Co., Lake Etling, 10.vi.1983, B.F. and J.L. Carr (1, CNCI); Cleveland Co., Norman (Oliver Woods), 30.vi.1969, W. Suter, nest in stump (1, LSAM), LSAM0142642; Marshall Co., 2 mi. SE of Willis, 5.iv.1968–24.vi.1969, willow swamp cedar, W. Suter (12, LSAM; 2, UAIC), LSAM0141740, LSAM0142383, LSAM0142623, LSAM0142629, LSAM0210485-LSAM0210491; Marshall Co., 2 mi. E Willis, Buncombe Creek Campground, 24.vi.1969, W. Suter, oak forest floor (1, UAIC); Marshall Co., University of Oklahoma Biological Station on Lake Texoma (Willis), 17.iv.1968, W. Suter (2, LSAM), LSAM0141724, LSAM0141725; Oregon: Grant Co., John Day, U.S. Hwy 30, 23.v.1959, Joe Schuh, on willow (1, CMNC); Columbia Co., 5 mi. N–2 mi. E of Burlington, app. 30, 7.x.1972, oak tree hollow debris and soil, E.M. Benedict (12, CMNC); Minam, 13.vi.1984, B.F. and J.L. Carr (1, CNCI); Dayton, Dorsey Gravel Bar, 8–22.vii.1962, K.M. Fender (5, CAS); Eugene, 29.v.1933, O.H. Swezey (2, CAS); Corvallis Co., 11.vi.1925, E.P. Van Duzee (1, CAS); Pennsylvania: Harrisburg, 20.vi.1920–27.v.1921, H.C. Fall Collection (3, MCZC), MCZ-ENT00727201 – MCZ-ENT00727203; Westmoreland Co., Jeannette, H.G. Klages (1, CNCI); New Cumberland, 30.iv.1910, H.C. Fall Collection (1, MCZC), MCZ-ENT00727198; Allegheny, F.C. Bowditch Collection (4, MCZC), MCZ-ENT00727023 – MCZ-ENT00727026; South Carolina: Clemson College, 20.vi.1936, O.L. Cartwright (1, CUAC); Georgetown Co., Georgetown, 4.iv.1957, Chamberlain (1, CUAC); Tennessee: Hamilton Co., 10 mi. S of Chattanooga, 8.iv.1981, P. Ramey, sweeping (1, MEM); Texas: Gonzalez Co., Palmetto State Park, 2.x.1973, A. Newton (3, MCZC), MCZ-ENT00727117 – MCZ-ENT00727119; Bandera Co., Bear Creek, 7 mi. NE of Bandera, 4.v.1999, S.M. Clark (4, UAIC); Berleson Co., 5.ii.1958 (1, UAIC); Briscoe Co., Quitaque Creek, 6.v.1970, on Salix (4, UAIC); Burnett Co., Inks Lake State Park, 21.v.1989, E.G. Riley (1, UAIC); Cameron Co., Brownsville, iv.1978, W.L. Johnson, in Boll Weevil Mitchell trap on cottonfield margin (1, MEM); Cameron Co., Brownsville, Palmetto Grove, 5–9.v.1978, A.E. and D.S. Lewis (1, UAIC); Crosby Co., White River, 3 mi. E of Crosbyton, 3.v.1970, C.W. O’Brien (1, UAIC); Dickens Co., White River Lake, 16.iv.–8.vi.1989, R. Morris (4, CMNC); Culberson Co., Guadalupe National Park, Choza Springs, 22.vii.1982, G.A.P. Gibson (1, CMNC); Grayson Co., Juniper Pt., L. Texoma (12 mi. N of Whitesboro), 6.iv.1969, W. Suter, litter around log (2, UAIC); Gonzales Co., Palmetto St. Park, 30.v.1977, E.G. Riley, blacklight (2, UAIC); Gray Co., Lake McClellan, 4.vii.1970, G.B. Marshall (2, UAIC); Eastland Co., 1 mile south of Eastland, 4.iv.1985, G.B. Marshall (1, UAIC); Harris Co., Hwy 59 at San Jacinto, 4.vi.1968 (1, UAIC); Jim Wells Co., Lake Corpus Christi, 7.vi.1954, Henry F. Howden (1, CMNC); Floyd Co., Lake Marvin, 14 mi. E Canadian, 23.iv.1970, L.B. and C.W. O’Brien (7, UAIC); Brazos Co., Peach Creek at Hwy 6, 8 mi. S of College Station, 13.iv.1987, R.S. Anderson (57, CMNC); Cameron Co., Southpoint Nursery (1 mile south of Southmost Ranch), 5–6.vii.1982, G.A.P. Gibson (2, CMNC); Jeff Davis Co., 3.4 km E of Fort Davis, 10–11.ix.1988, R.S. Anderson, Berlese funnel trap with mixed hardwood litter (6, CMNC); Jeff Davis Co., 5 mi. N of Fort Davis, 14.vii.1956, Henry and Anne Howden (1, CMNC); Red River Co., 2 mi. NW of Talco, 8.vi.1965, C.W. O’Brien, on Salix nigra var. vallicola (6, UAIC); Val Verde Co., Langtry, 4.xi.1982, J. Huber and A. Gonzalez, sweep along river edge (2, CMNC); Kerr Co., Kerrville, 4.iv.–22.iv.1959, Becker and Howden, beating Salix (92, CNCI); Brewster Co., Lajitas, 19.v.1959, Howden and Becker (1, CNCI); El Paso, Fenyes (1, MCZC), MCZ-ENT00727144; Bandera Co., Vanderpool, 18.iv.1987, B.F. and J.L. Carr (5, CNCI); Utah: Provo, Utah Lake, 4.vii.1964, L.B. and C.W. O’Brien (2, UAIC); Sevier Co., Sevier River, 24.vi.1966, W. Gagne (1, UAIC); Wayne Co., Torrey (2 mi. E), 2.viii.1968, Anne T. Howden (2, CMNC); Washington Co., 7 mi. W Sta. Clara, 8.iv.1963, L.B. and C.W. O’Brien, on Salix laevigata Bebb (5, UAIC); Washington Co., 7 mi. E Gunlock, 8.iv.1963, C.W. O’Brien, on Salix laevigata Bebb (1, UAIC); Washington Co., Virgin – 3400’, 7.iv.1963, C.W. O’Brien, swept from Salix (3, UAIC); Washington Co., St. George, 8.iv.1963, C.W. O’Brien, on Salix exigua (1, UAIC); Washington Co., Virgin, 10.iv.1963, L.B. and C.W. O’Brien, at night on fruiting Populus fremontii (1, UAIC); “Ut.”, Liebeck Collection (1, MCZC), MCZ-ENT00727014; Fort Douglas, Wickham (2, MCZC), MCZ-ENT00727032, MCZ-ENT00727033; Virginia: Accomack Co., Deep Creek, Dismal Swamp, 5.v.1951, G.H. Nelson (1, CMNC); Fairfax Co., Quirsfeld (4, CAS); Washington: Snohomish Co., Sulton, 11.v.1933, R.A. Flock (2, UAIC), UAIC1072872, UAIC1072873; West Virginia: Cabell Co., Green Bottom Wildlife Management Area, 25.iv.1996, L. Torres-Miller (1, CMNC); Wyoming: Park Co., junction between Hwy 120 and Hwy 296, 27.vi.1982, B.F. and J.L. Carr (1, CNCI).
Diagnosis. Length (2.0–2.9 mm). Scales on pronotum usually not uniform in colour, with discoloured central and lateral bands. Metaventrite and metepisternum with scales relatively sparse not obscuring underlying cuticle. Distinct swelling at the tip of last ventrite of males (no longitudinal carina at the middle of ventrite; often reduced or absent in smaller specimens). Apex of aedeagus rounded and coming to a distinct point, with two prominent tufts of setae. Internal sac with protruding basal structures. Widespread across North America (absent from California and southern Oregon).
Distribution in North America. This is the most widespread species of Ellescus in North America, with records across Canada and the United States of America (except for California and the southern tip of Oregon). Records from southern Texas and southern Arizona suggest that the species may also be present in Mexico.
Variation. This species is extremely variable in size and colour across its range (see Fig. 9), and most instances of misidentification of Ellescus in North America previously were cases of confusion with this species.
Natural history. This species has been collected on a wide variety of Salix and Populus species, including Populus tremuloides, Populus fremontii, Salix brachycarpa var. brachycarpa Nuttall, Salix nigra var. vallicola, Salix exigua, and Salix laevigata Bebb. Blatchley and Leng (Reference Blatchley and Leng1916) note the rearing of E. ephippiatus from the willow galls of Rhabdophaga strobiloides (Osten Sacken, 1862). LeConte (Reference LeConte1876) also report the rearing of E. ephippiatus from the galls of Rabdophaga rigidae (Osten Sacken, 1862) on Salix interior Rowlee (= S. longifolia). Hight (Reference Hight1988) reported collecting the larvae of E. ephippiatus from the terminal bud of Lythrum salicaria L.; however, this identification is questionable given the lack of other such reports and the strong association of the family with members of Salicaceae. A large series from northern Oregon, United States of America, were taken from oak tree hollows, debris, and soil. This species is known to be attracted to lights (Ciegler Reference Ciegler2010).
Remarks. Despite being described from specimens collected in Indiana, United States of America, label data for a syntype of E. ephippiatus deposited in the Carl Schönherr collection (NHRS) read as follows: “epphippiatus [sic]/ Say./ Connecticut./Say” (Prena Reference Prena2018). The locality (Connecticut) appears to have been erroneously introduced by Say during preparation of a list of specimens shipped to Schönherr, or by Schönherr who may have misinterpreted Say’s list. A handwritten copy of the list of specimens sent by Say is preserved in the Academy of Natural Sciences of Philadelphia (ANSP) and suggests that the Connecticut collection locality for Phyxelis rigidus (Say, 1831) may have been erroneously mistaken for the collection locality for the E. ephippiatus, which follows subsequently in the same list.
Conclusion
The species of Dorytomus in North America were reviewed by O’Brien (Reference O’Brien1970) and the North American endemic genus Proctorus was recently revised by Lewis and Anderson (Reference Lewis and Anderson2022); the addition of the current study brings our understanding of the taxonomy Ellescini in North America to new heights and paves the way for more comprehensive taxonomic studies. Although Ellescus required special attention in North America (hence the current study), a comprehensive world revision is still needed. Material representing all Ellescus species was examined; however, type material for some key Old World species remains unexamined or unknown, thus prohibiting a proper revision of the entire genus. A world revision of Ellescus will be covered in a future study of the genus along with a preliminary molecular phylogeny of the tribe.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2023.2.
Acknowledgements
The authors thank Dr. Michael Košťál (Slovakia), an independent weevil researcher who graciously collected and mailed fresh specimens for use in this research. The authors also thank Roger Bull and Anna Ginter (Molecular Lab, Canadian Museum of Nature, Ottawa, Ontario, Canada) for their support during the course of this research. Dr. Jens Prena is thanked for providing additional comments on the E. ephippiatus syntype. They are grateful to numerous curators, collection managers, and independent researchers for providing loans of specimens, and also thank Dr. Andrew Smith (Canadian Museum of Nature), Dr. Patrice Bouchard (Canadian National Collection of Insects, Ottawa, Ontario), and Dr. Derek Sikes (University of Alaska Museum Insect Collection, Fairbanks, Alaska, United States of America) for their constructive comments on the manuscript. This research was funded by the Canadian Museum of Nature and the University of Ottawa.